Sed Pet Week V
1/31
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
Fault breccia
A rock with poor sorting, and angular clasts that are mostly of the same composition. When you have something like a strike-slip boundary, lithologies are similar on both sides, which is why we see this. Clasts might be granite, chert, basalt
moderate amount of matrix
Solution collapse
A rock with poor-moderate sorting, and angular clasts that are of similar composition. What makes this different from a fault breccia is the type of clasts and their sorting; generally, they are carbonate or sedimentary clasts, the result of a cave collapse that dropped rock without sorting or rounding it. Clasts may be lighter in color and more fragile
small-moderate amount of matrix
Direction of wave/dune ripples
Generally face concave-up, like how sand dunes migrate. If you were to find a bed sticking out of the surface that shows ripples facing concave-down, that might indicate the beds have been tilted upside down
Why there is an oxygen minimum near the seafloor?
Algae/small organisms die and float down to the seafloor and undergo aerobic decay, using up oxygen and creating a dead zone. However, these nutrients are released into the water and upwelled to the surface, creating productivity at higher levels (positive feedback loop)
Bathymetric sill (phosphorite formation)
An underwater berm that sticks up and impedes sediment/water movement, creating an anoxic water basin that is more dense than the upper water levels. On the seafloor in that basin, organic-rich mud is created and continues to decay, but because there is little oxygen, sulfates are reduced into sulfides. More phosphate is released into the pore spaces between grains, allowing it to become very saturated. Small crystals of apatite will grow and replace carbonate grains, where they are concentrated and fuse into phosphorites
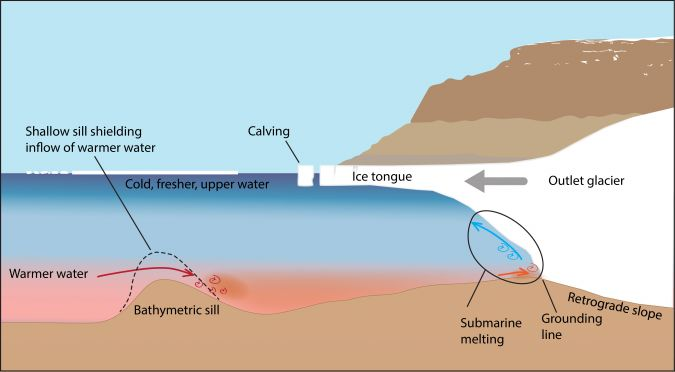
Where does material for upwellings come from?
Bathymetric sill basins; material from organic decay is pushed upwards due to wind and wave movement at the surface, creating productivity there
Why do carbonates become less present when moving deeper underwater?
Because at the surface, wave action and oxygenation drive away CO2, driving up the pH and precipitating more calcite/aragonite. The water is also warmer here. Moving down, there is less wave action, causing CO2 to become more concentrated. This lowers pH, making the water more acidic and causing carbonates to dissolve. The water is also colder
Autochthonous
“In-place;” rocks forming in their current environment without transport, weathering, or erosion. Carbonates are a good example
Allochthonous
'“transported;” rocks that have been brought from another place rather than forming in their current environment. Include weathering, sorting, erosion
What are the textures of carbonates dependent on?
Because carbonates are organically derived, the textures of carbonates are dependent on the nature of the contributing skeletal producers
Biofacies
The type of organisms contributing to the formation of a rock
Lithofacies
The type of sediment contributing to the formation of a rock
Micrite
Carbonate (bioclastic) mud; the amount present in a carbonate is indicative of wave action and depositional environment. Carbonate formation can be very rapid or not happen at all, depending on organic presence. It is very sensitive and can switch quickly
Why carbonates are sensitive to diagenesis?
Due to high reactivity; textures can be modified entirely and minerals can alter to other forms
Equilibrium constant (K)
ratio of products to reactants at equilibrium, indicating how far a reversible reaction proceeds
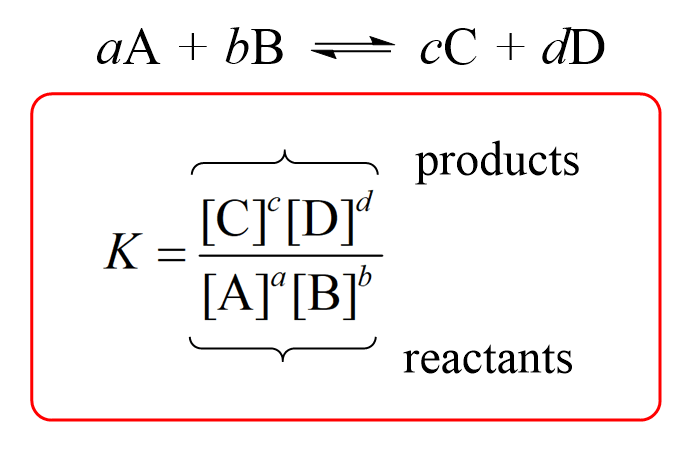
If the equilibrium constant is small (carbonate formation)
concentration of products is smaller than amount of reactants
If the equilibrium constant is large (carbonate formation)
concentration of products is larger than amount of reactants
Processes affecting amount of dissolved CO2
agitation of water (wave action)
temperature
rate of evaporation
rate of biogenic removal via photosynthesis
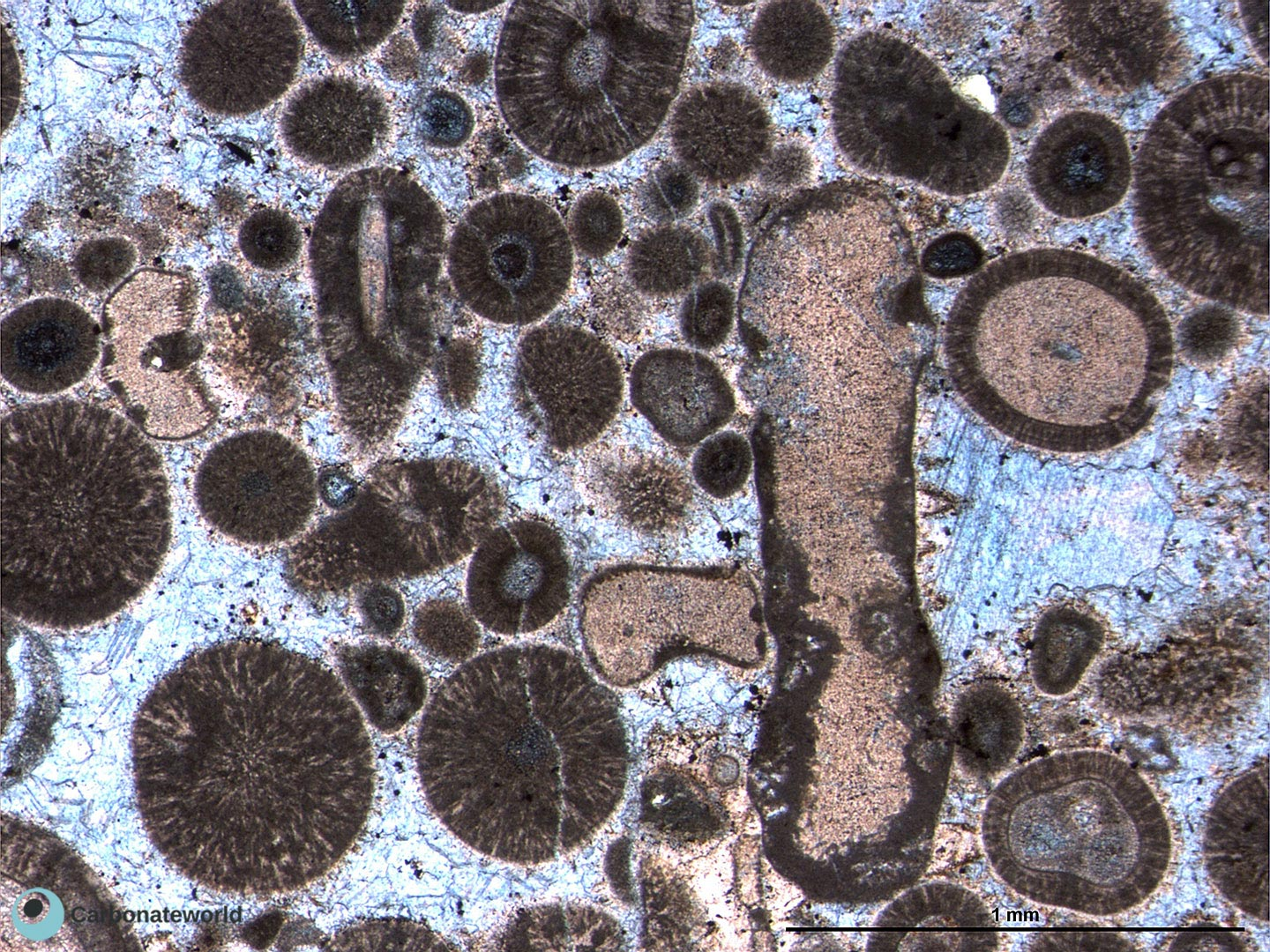
Carbonate grain types
intraclasts
ooids
bioclasts
peloids
aggregated lumps (many chunks of skeletons formed into one grain)
oncoids (irregular, large ooids, not as rounded)
Bioclast types
Sheathed/spicule skeletons (matter held together by organic tissue; sponges)
segmented skeletons (crinoids)
branched skeletons (acropora coral)
chambered skeletons (gastropods, foraminifera)
massive skeletons (stromatoporoids)
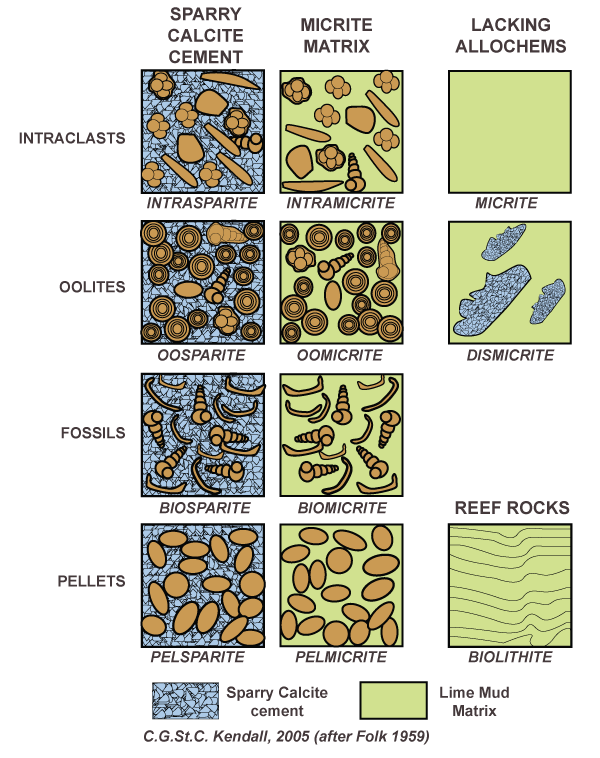
Folk classification scheme
based on relative abundance of intraclasts, oolites, fossils, and pellets. Also based on amount of cement present
the rock is either a sparite or micrite
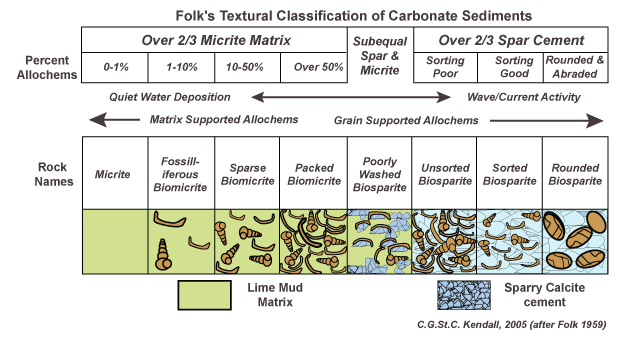
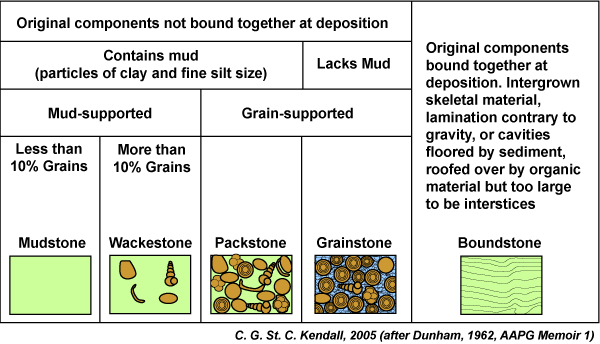
Dunham classification scheme
Based on presence of carbonate mud. If there is none, the rock is a grain-stone (high wave energy removes mud particles). Primary porosity is either air space or cement.
If there is mud present, grains are either floating in the matrix or grain supported. Wackestone is matrix-supported, packstone is grain-supported
Boundstone is a carbonate in which the constituents of the rock are cemented together during deposition, creating a very tightly packed rock with few distinguishable grains
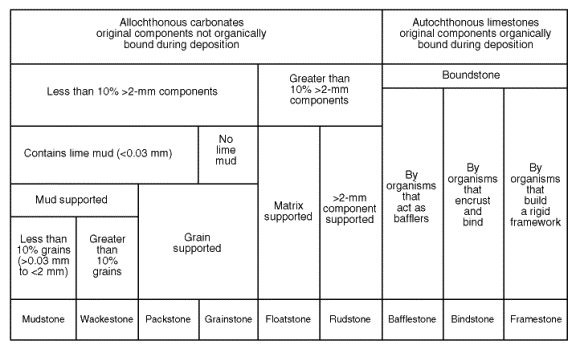
Modification of Dunham’s scheme
recognition of the fact that some carbonates have grains larger than sand, like gravel or pebbles. If >10% of the grains are gravel sized or larger, the rock is no longer a boundstone, but a floatstone or rudstone.
Boundstones are divided into categories: bafflestone, bindstone, and framestone
Sources of carbonate mud
Breakdown of calcareous green algae (Penicillus) and skeletal debris. When the organism dies, its spicules dissolve into the seafloor sediment
Whitings
Micritic envelopes

Whitings
Inorganic precipitation of needle-like carbonate mud particles. In the ocean, conditions are such that carbonates can be precipitated directly from the sea, creating cloudy white spots that can be seen from the air
Micritic envelope
An organism bores into a shell, creating a dark carbonate film around it. This is done by a type of algae (endolithic boring algae) that produces carbonate mud as it digs into the shell
Carbonate sedimentation in the ancient rock record vs. now
Carbonate shows low rates of sedimentation (<1 meter per millions of years) in ancient rock record. In modern conditions, however, carbonate sedimentation rates reach as high as 3+ meters in just 7000 years. This shows how quickly the conditions required for carbonate formation can change
Carbonate reef
Buildup of carbonate that is a lithified, wave-resistant structure. Behind this buildup, closer to the mainland, you see a shallow lagoon with higher salinity than the open ocean. Organisms here will vary according to salinity and temperature tolerance
South coast of Florida is a good example
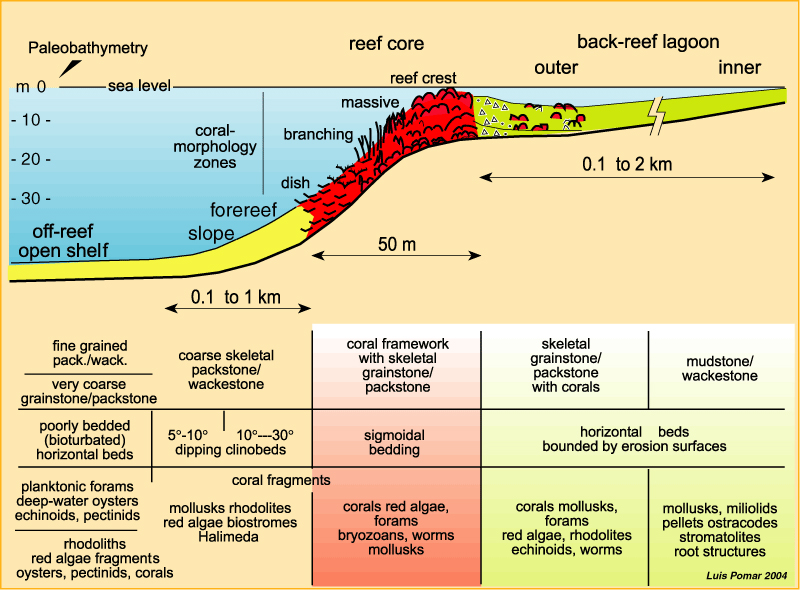
Carbonate reefs are optimal environments for carbonate-forming organisms. True?
True; this is because there is a lack of siliciclastic constituents (clay). The water is not cloudy, so photosynthesis is promoted. Very clear blue water with white sediment
Marine regression
Water recedes from shore; Deep water sediments (limestone and shale) move further back as the water level lowers. Upward coarsening
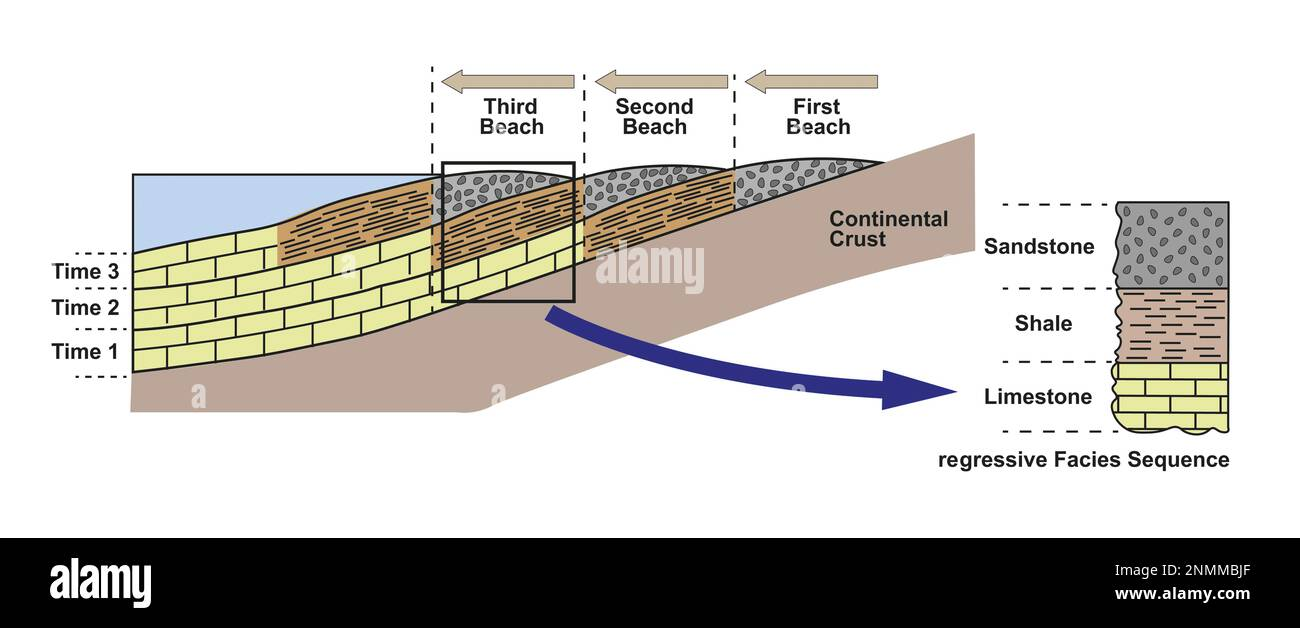
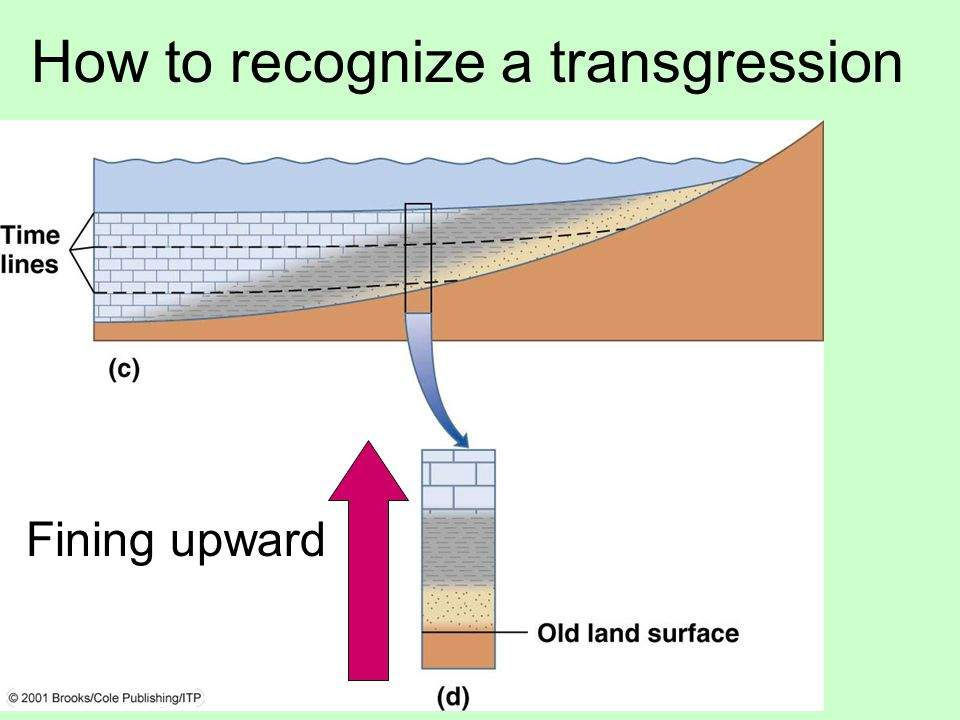
Marine transgression
Water moves up-shore; Deep water sediments (limestone, shale) move further up onshore with rising water level. Upward fining
In the burial of a shell, aragonite will often dissolve and be replaced by calcite. True?
True. Aragonite is more soluble than calcite, so it will dissolve sooner as burial/lithification continues. Because the micrite the shell is buried in is rich in calcite, the cement will precipitate to fill the space of the shell, replacing it
common with gastropods and bivalves