Identification of gases
1/5
Earn XP
Description and Tags
part 3 of 12.5 Identification of ions and gases
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
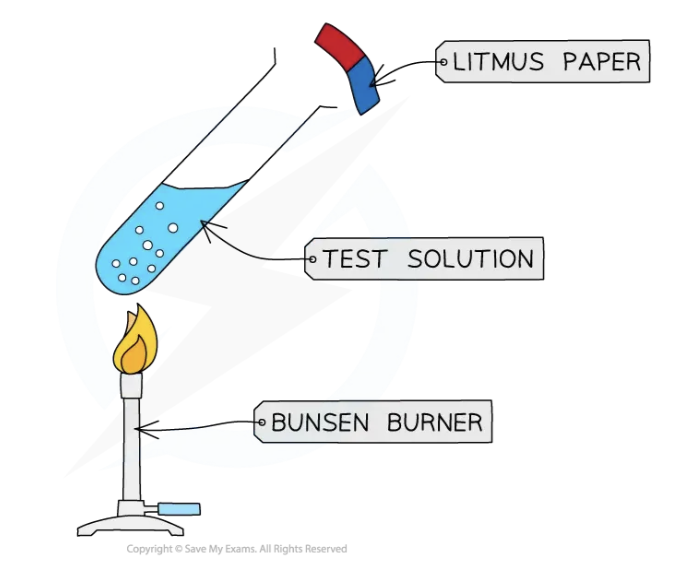
Test for ammonia gas: NH3
Ammonia turns damp red litmus paper blue
Hold the litmus paper near the mouth of the test tube, but be careful to avoid touching the sides of the test tube
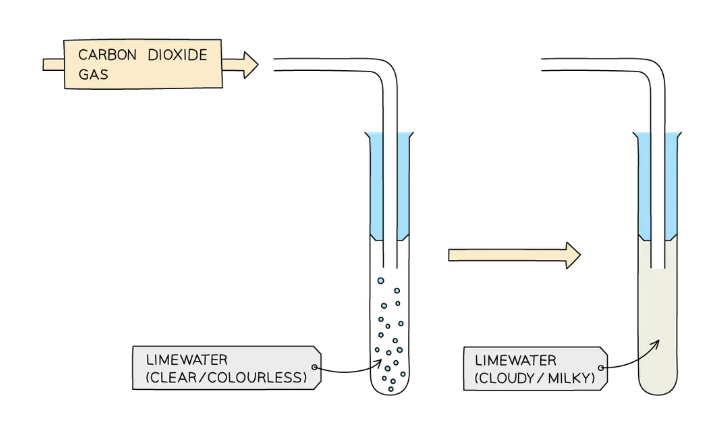
Test for carbon dioxide gas: CO2
bubble the gas through an aqueous solution of limewater (calcium hydroxide)
If the gas is carbon dioxide, the limewater turns cloudy white
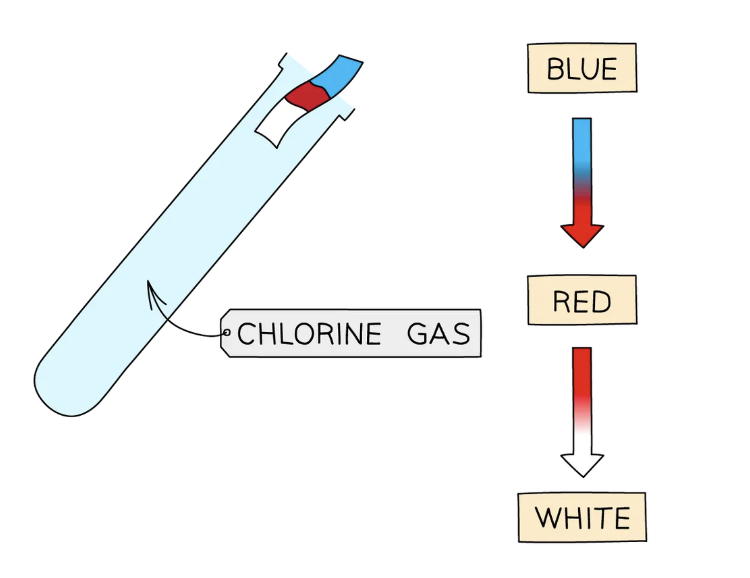
Test for chlorine gas: Cl2
If chlorine gas is present, damp blue litmus paper will turn red and then be bleached white
Chlorine should always be handled in a fume cupboard due to its toxicity
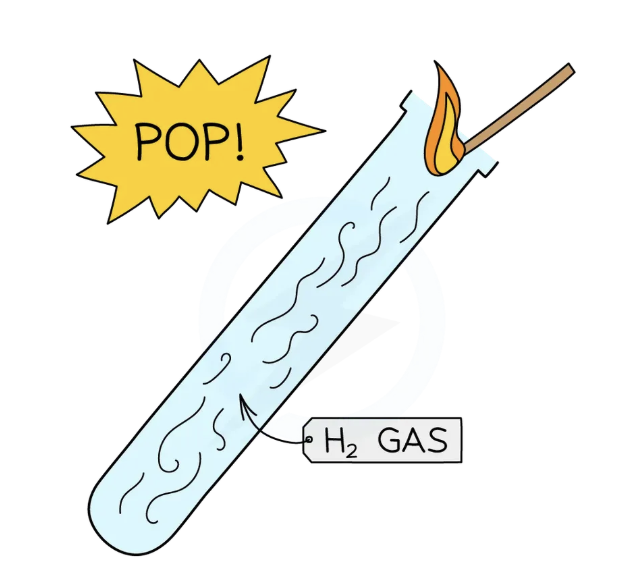
Test for hydrogen gas: H2
hold a burning splint at the open end of a test tube of gas
If the gas is hydrogen it burns with a loud “squeaky pop” which is the result of the rapid combustion of hydrogen with oxygen to produce water
Be sure not to insert the splint right into the tube, just at the mouth, as the gas needs air to burn
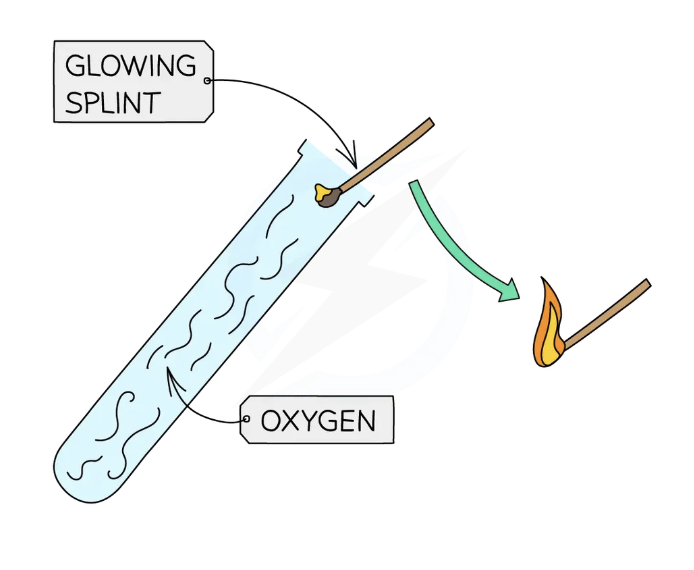
Test for oxygen gas: O2
place a glowing splint inside a test tube of gas
If the gas is oxygen, the splint will relight
Test for sulfur dioxide gas: SO2
Bubble the gas through an acidified solution of potassium manganate(VII)
If the gas is sulfur dioxide, the potassium manganate(VII) changes from purple to colourless