enthalpy
5.0(1)
5.0(1)
Card Sorting
1/29
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
1
New cards
What is an exothermic reaction
a reaction that gives out thermal energy to the surroundings
2
New cards
how to identify an exo reaction
a rise in temp in surroundings
3
New cards
what happens to temp of system in an exo reaction
decrease in temp
4
New cards
what is an endothermic reaction
reaction that takes in thermal energy from the surroundings
5
New cards
how to identify an endo reaction
fall in temp of surroundings
6
New cards
what happens to temp of system in endo reaction
temp rise
7
New cards
what is the law of conservation of energy
energy cannot be created or destroyed only transferred
8
New cards
give 2 examples of types of endothermic reactions
dissolving, neutralisation
9
New cards
give 2 examples of types of exothermic reactions
combustion , displacement
10
New cards
what do energy level diagrams show
the relative energy and changes in energy of reactants and products in a reaction
11
New cards
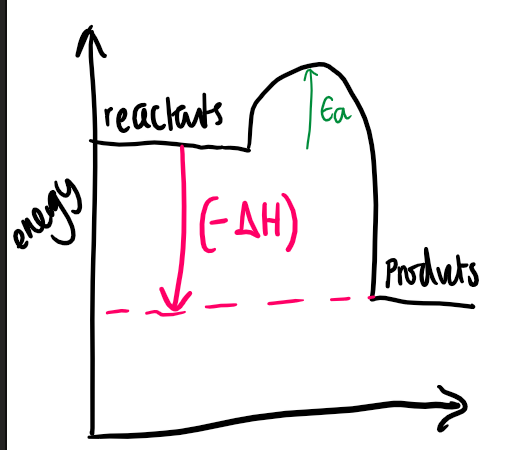
what type of reaction is shown
exothermic
12
New cards
what is activation energy
minimum amount of energy the reactants need to collide with each other and react
13
New cards
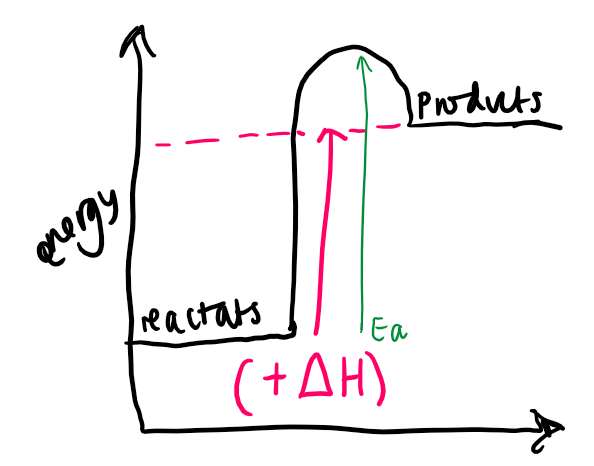
what type of reaction is shown
endothermic
14
New cards
where do we draw the line from and to for ΔH on the E.L. diagram
from the reactants to the products
15
New cards
where do we draw the line from and to for Ea on the E.L. diagram
from the reactants to the top of the curve (to direction of products)
16
New cards
is the ΔH +ve or -ve for each reaction and why
\+ve for endo and -ve for exo bc represents the enrgy either being released (exo) or taken in (endo)
17
New cards
do products have more or less energy than reactants for both
less for exo and more for endo
18
New cards
what is the symbol Q + units
heat energy change , J
19
New cards
what is the symbol m +units
mass of substance being heated, g
20
New cards
what is the symbol + units for ΔH
enthalpy change (heat transfer/mole) , KJ/mol
21
New cards
what is the symbol + units for c
heat capacity → 4.18 , J/g/°C
22
New cards
what is the symbol + units for ΔT
temp change, °C
23
New cards
what is the equation linking heat change , heat capacity, mass and temp change
Q= mc ΔT
24
New cards
what is the equation linking mass, moles and mr
moles=mass/mr
25
New cards
what equation links enthalpy change, moles and heat change
ΔH= -Q/moles
26
New cards
what is the density of water
1g/cm3
27
New cards
how do you measure energy transferred in a reaction for displacement, dissolving and neutralisation
take start temp of 2 reactants , mix in polystyrene cup inside a beaker w a lid (cotton wool optional) , stirring , record most extreme end temp (max or min)
28
New cards
how do you measure energy transferred in a reaction for combustion
weigh mass of spirit burner and lid and vol of water , then burn the fuel in spirit burner placed under a copper can filled w water, getting heated by the flame. weigh spirit burner and lid again and vol of water. calculate delta H
29
New cards
how to improve diss,disp,neut experiment
cotton wool in beaker for insulation of heat ,lid reduces heat loss by evaporation , be consistent stirring
30
New cards
how to improve combustion experiment
use a screen to reduce draughts, dont do next to a window , so amap heat goes to heating water,, make sure to be consistent with whether you weigh the lid or not