Chemistry Memorize Band of Stability
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Beta Plus Emission and Electron Capture
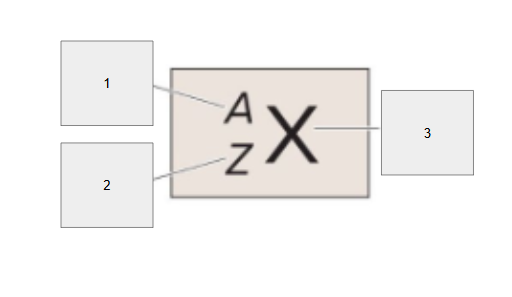
What does box 1 state? What is below the Band of Stability?
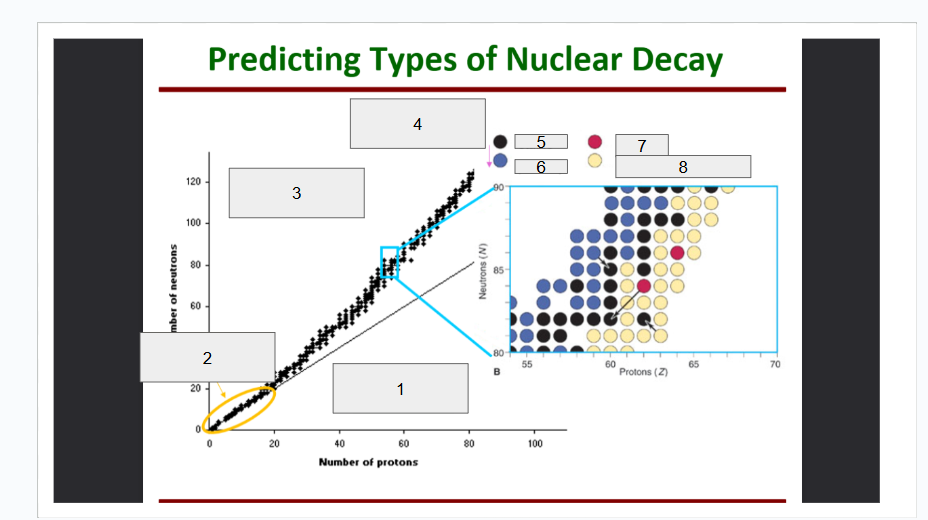
Stable Elements (n/p) = 1
What does box 2 state?
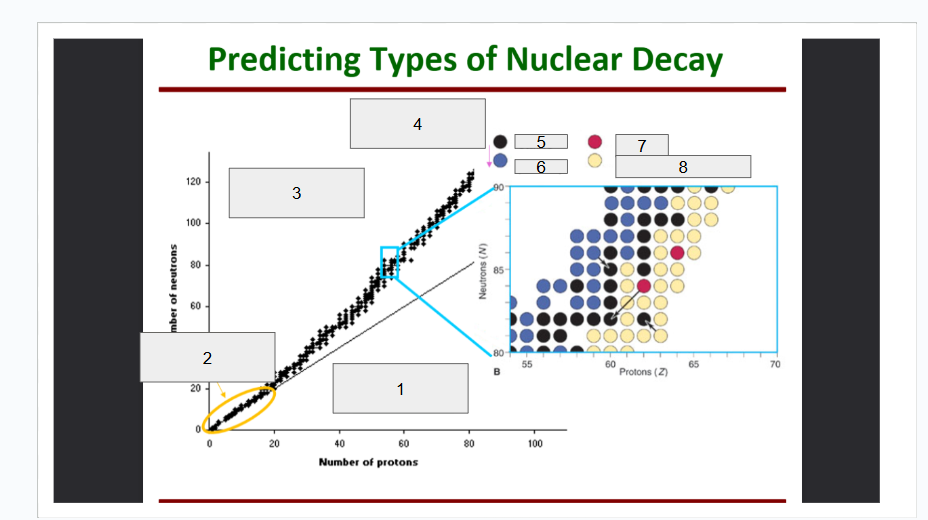
Beta Minus Decay
What does box 3 state?
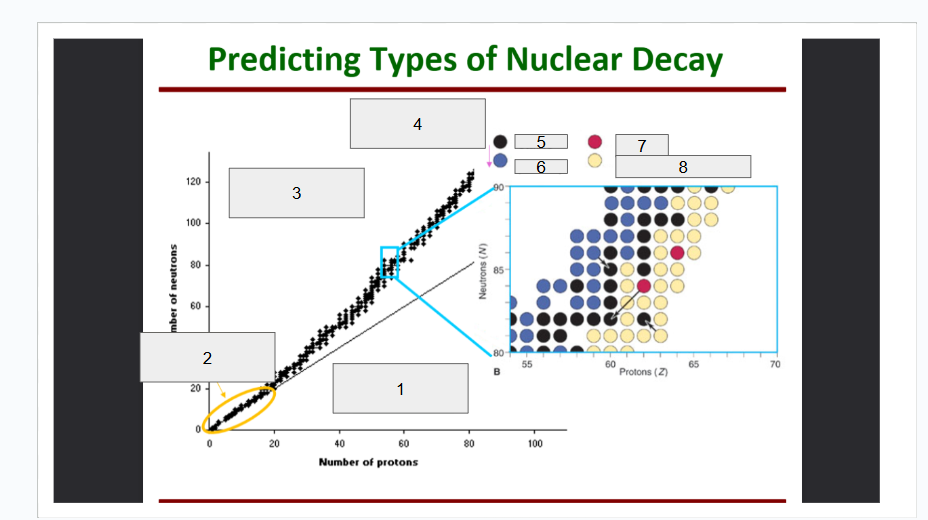
Alpha Decay
What does box 4 state?
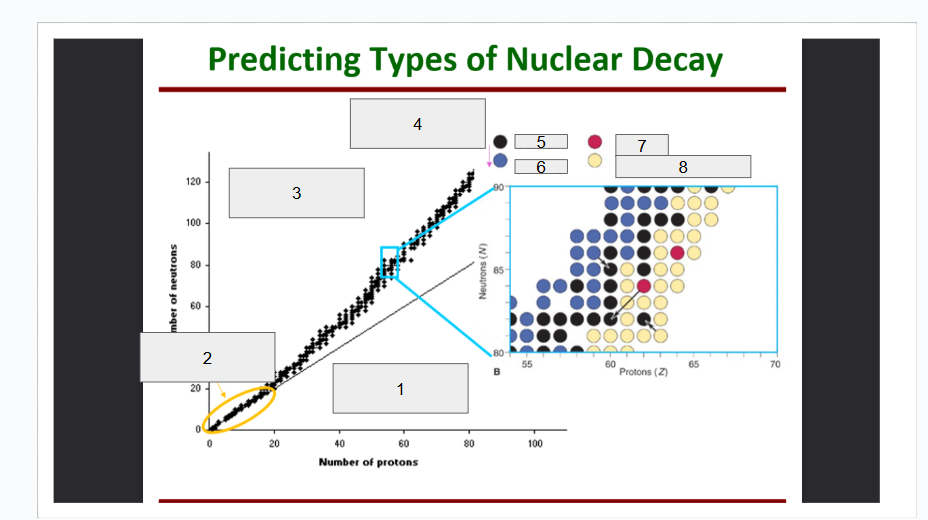
Stable
What does box 5 state?
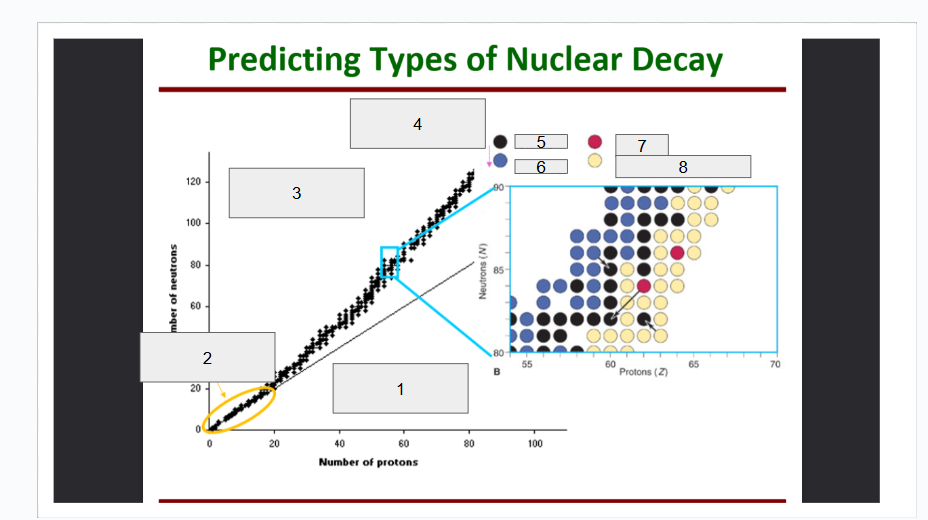
Beta Decay
What does box 6 state?
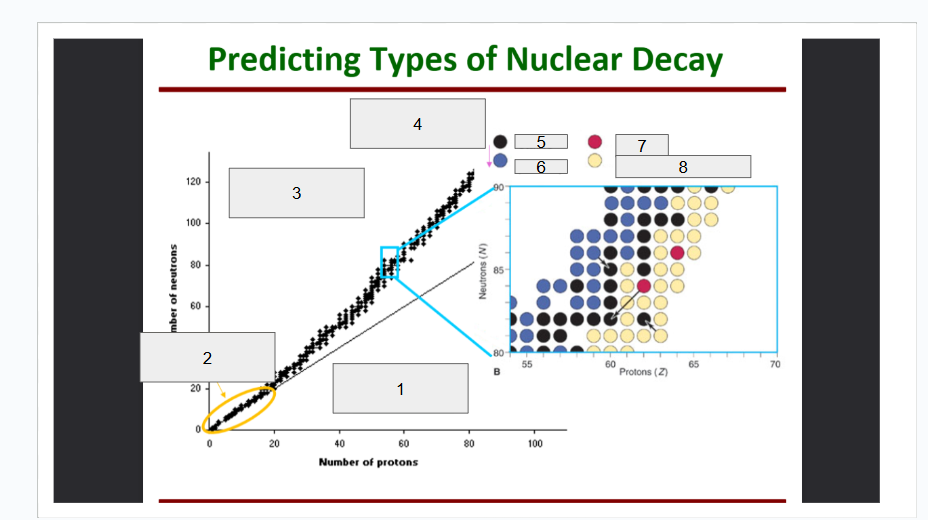
Alpha Decay
What does box 7 state?
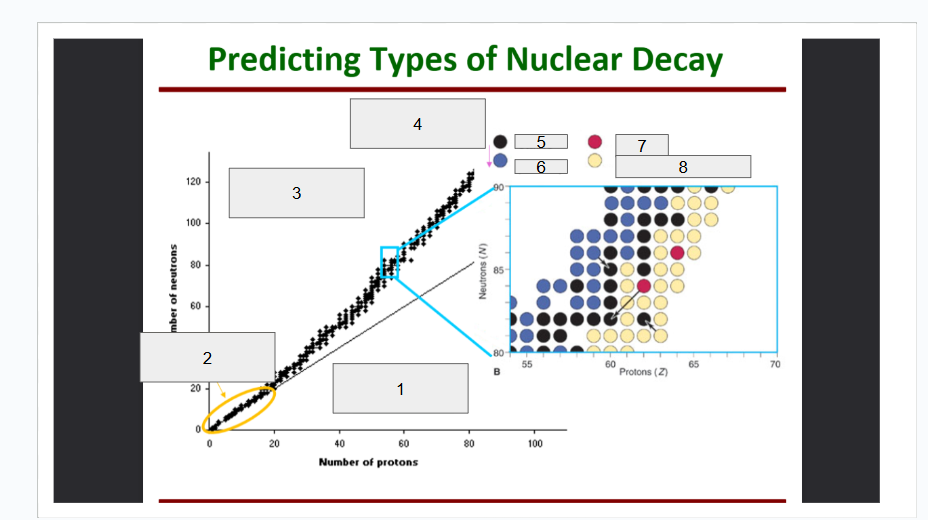
Beta Plus Emission and Electron Capture
What does box 8 state?
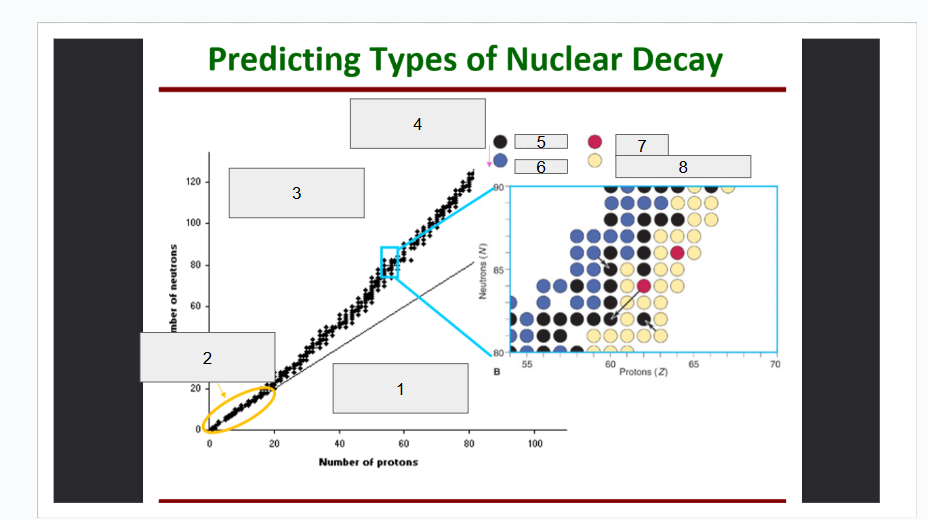
n/p too large
Increase number of protons. Beta decay: neutron decays to proton plus a beta particle
p/n too large
Increase neutrons, or reduce protons. Positron decay or electron capture
Above the band of stability
Alpha decay
Radioactive And Tends To Show Alpha Decay
Every element beyond Bi (atomic number, Z > 83) is
Stable
Lighter nuclides with N:Z = 1 (example: He, C, O, Ne, Ca) are
Neutron Number
What is box 1?
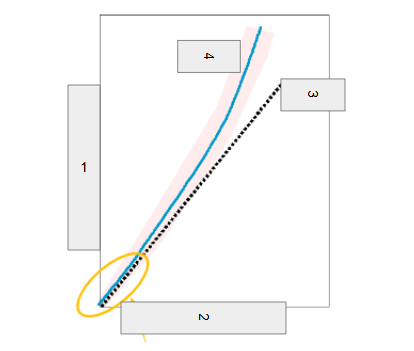
Proton Number
What is box 2?
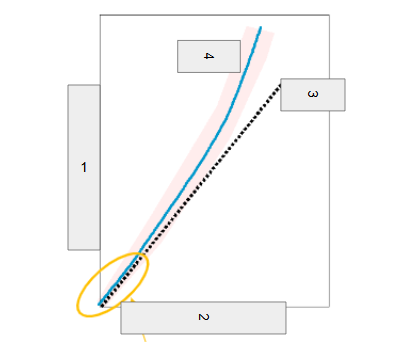
n=p
What is box 3?
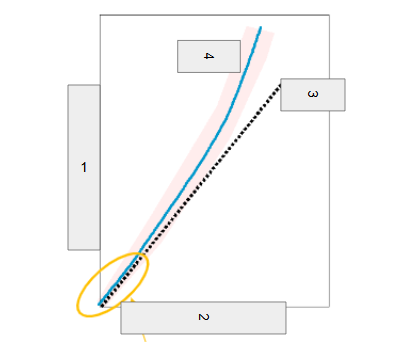
Stable
What is box 4?
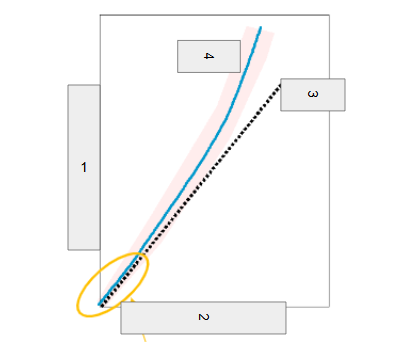
Band of Stability
What does the image represent
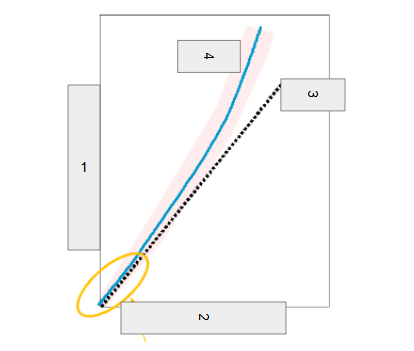
Mass number (p^+ + n^0)
What is box 1?
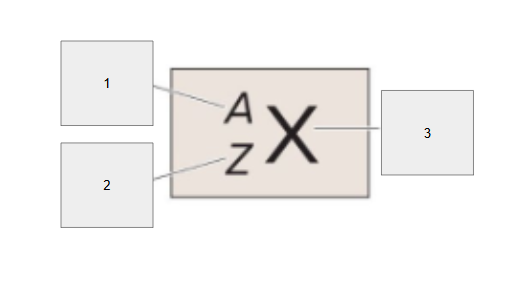
Atomic number (p^+)
What is box 2?
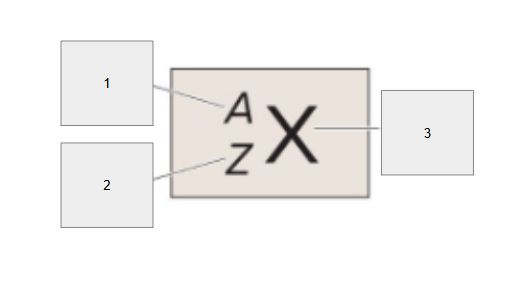
Atomic Symbol
What is box 3?