Atomic Mass, Atomic Number, Ions and Isotopes
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
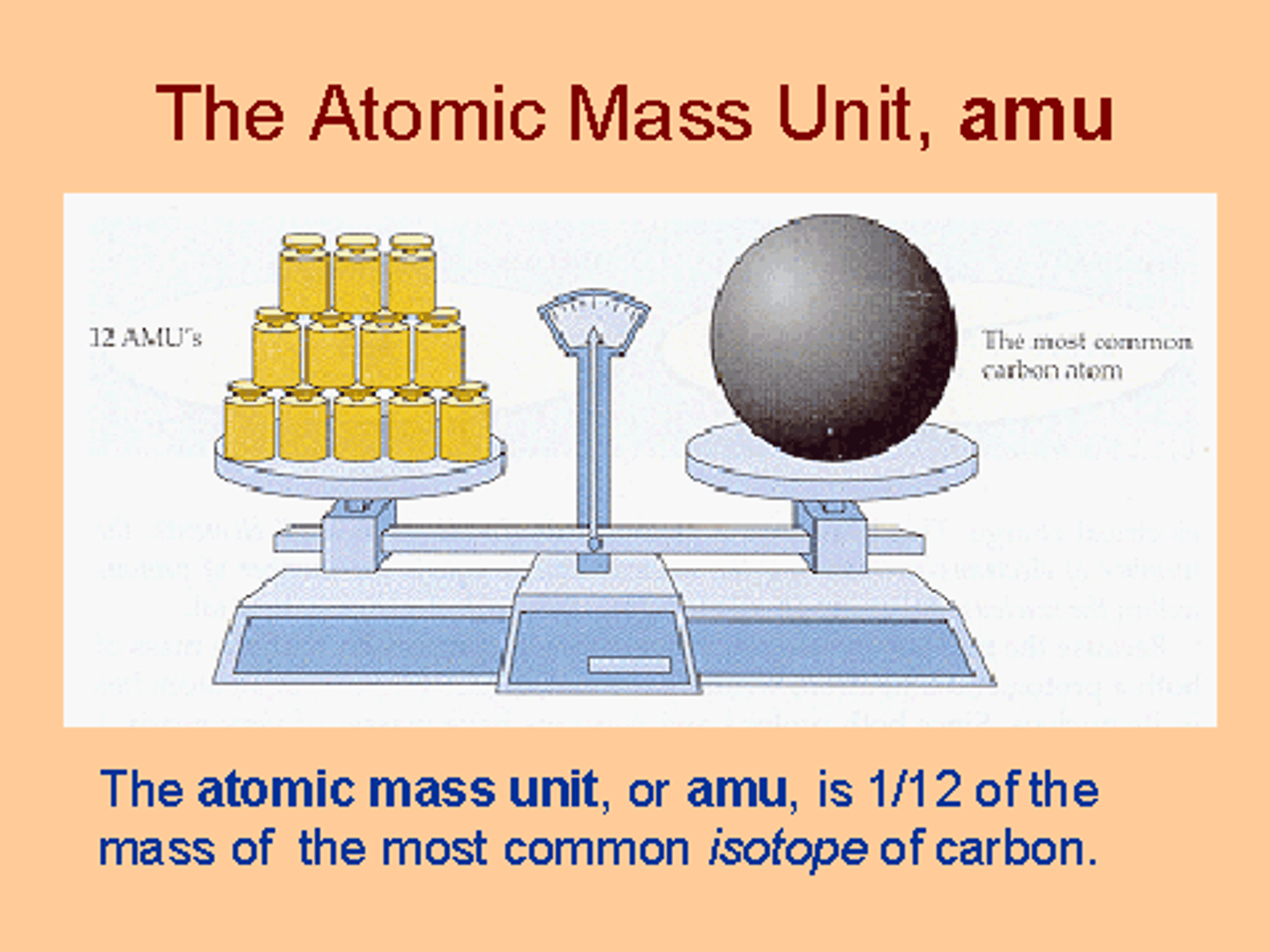
Atomic mass unit
(amu) A small mass unit used to describe the mass of very small particles such as atoms and subatomic particles
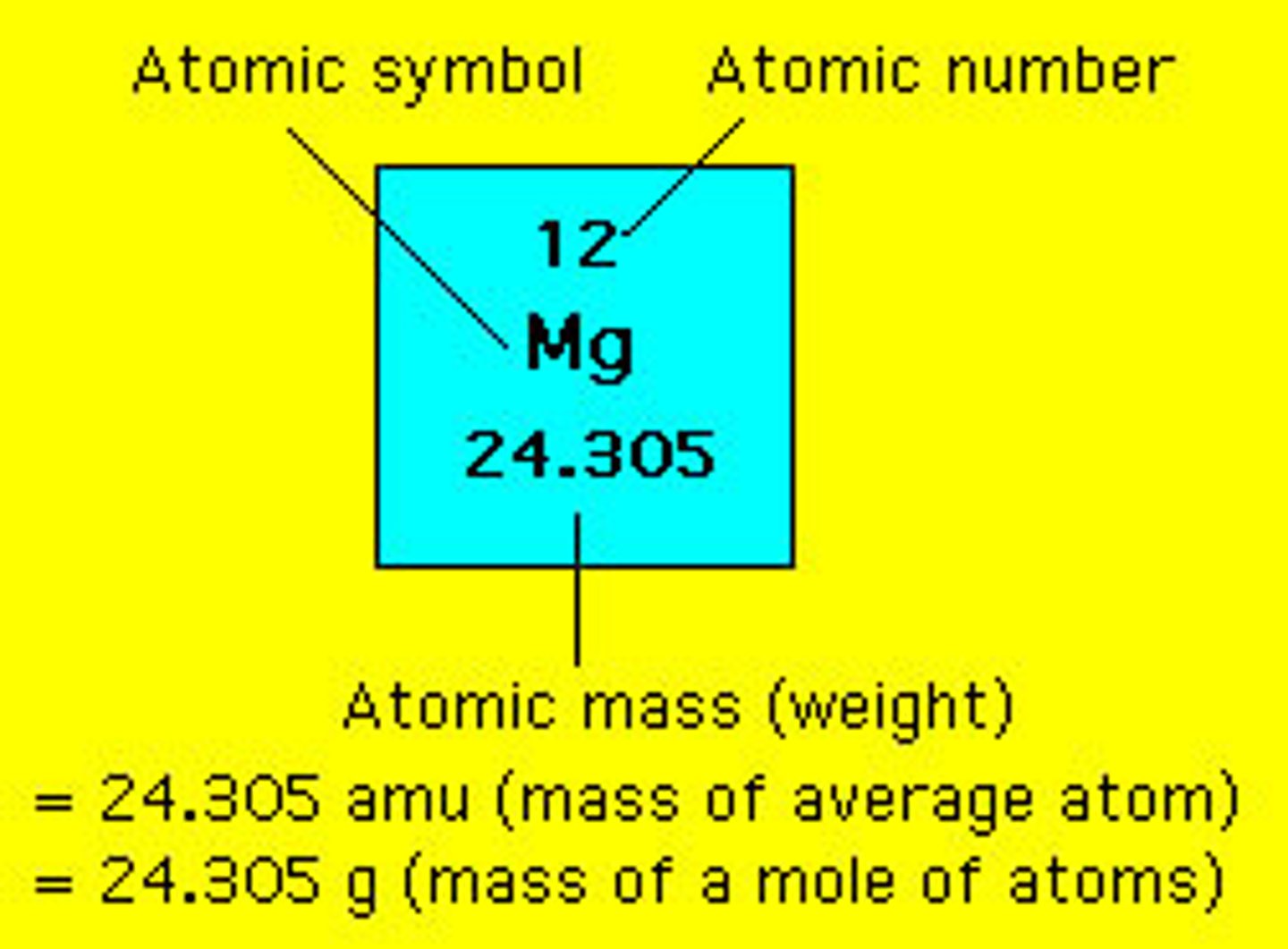
Atomic number
Number of protons in the nucleus of an atom
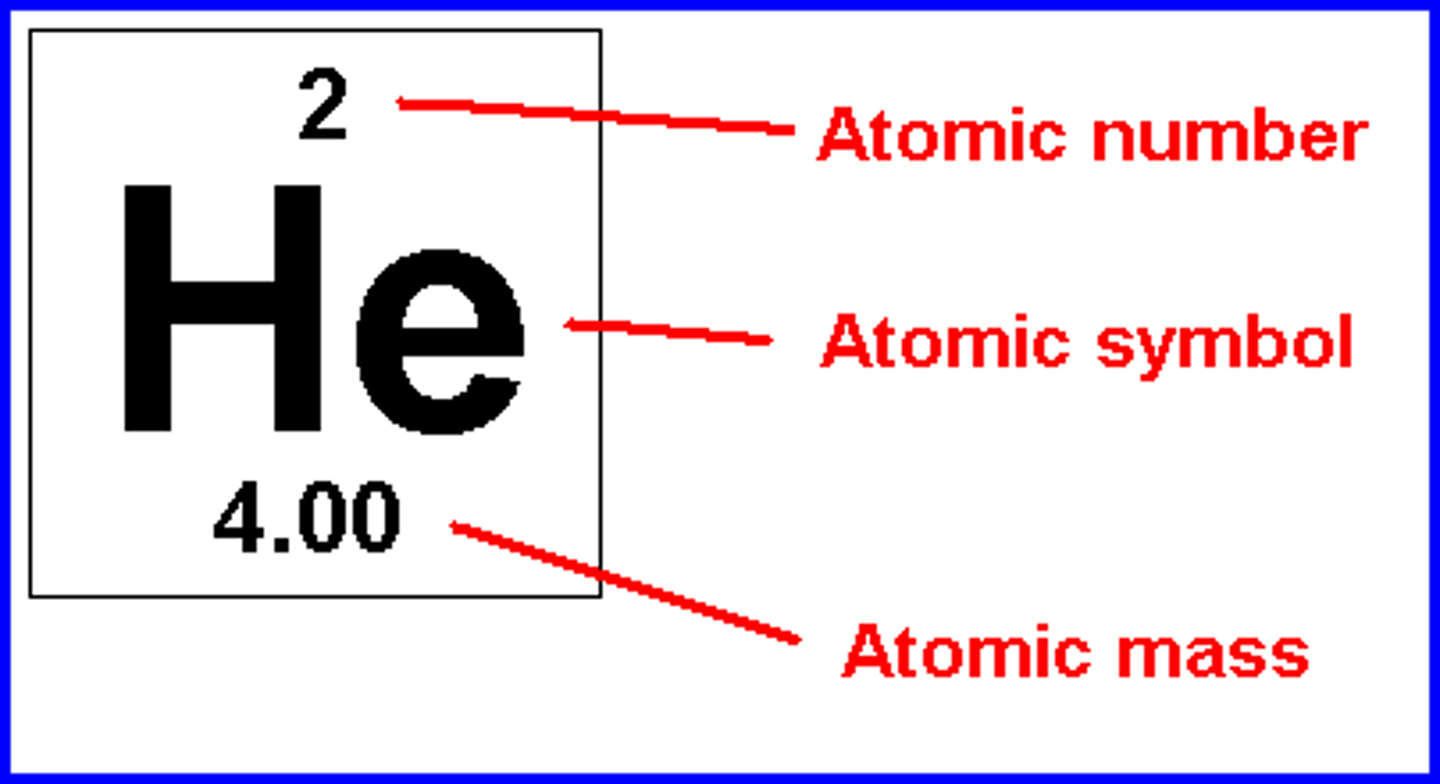
Average atomic mass (amu)
*The mass of a single atom
Net charge
How many protons and electrons "balance out"
Zero net charge
Atoms with an EQUAL number of protons and electrons
# Protons = # Electrons
Positive net charge
Atoms with more protons than electrons
# P > # E
Atoms with a positive charge are called: Cations
Negative net charge
Atoms with less protons than electrons
# P < # E
Atoms with a negative net charge are called: Anions
Ions
Atoms with a charge
Atoms become ions by gaining or losing electrons
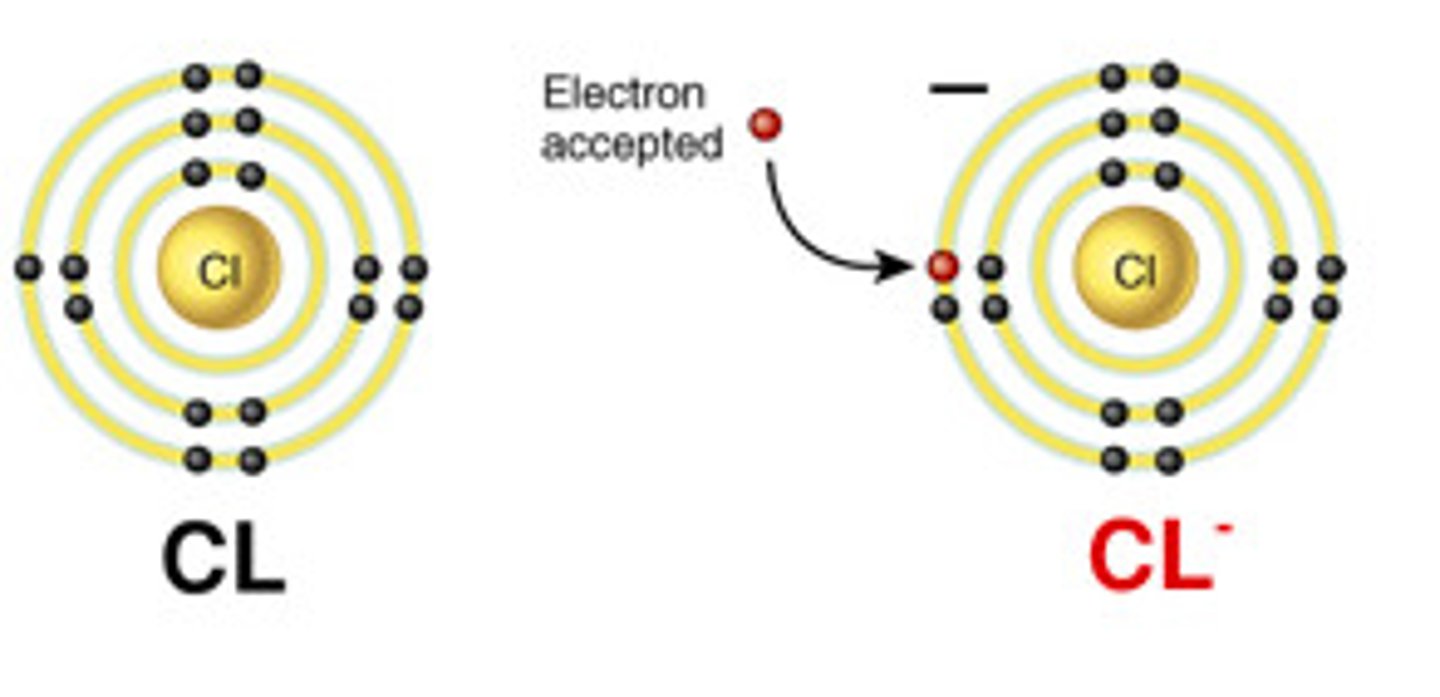
Anion
Atoms that have GAINED electrons
Electrons have a NEGATIVE CHARGE. When an atom gains electrons, there are more electrons than protons
+1 charge
The atom (ion) has 1 MORE PROTON than electrons
-1 charge
The atom (ion) has 1 MORE ELECTRON than protons
+2 charge
The atom (ion) has 2 MORE PROTONS than electrons
-2 charge
The atom (ion) has 2 MORE ELECTRONS than protons
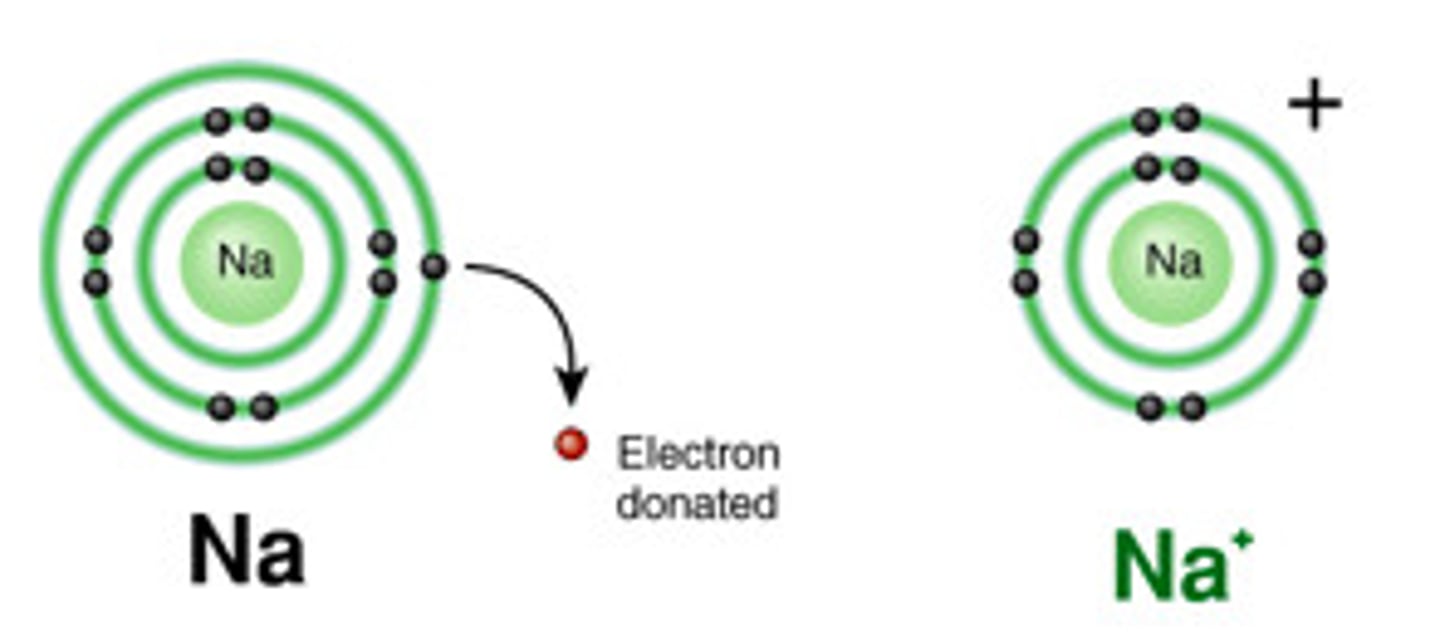
Cation
An atom that LOST electrons and therefore has a positive charge.
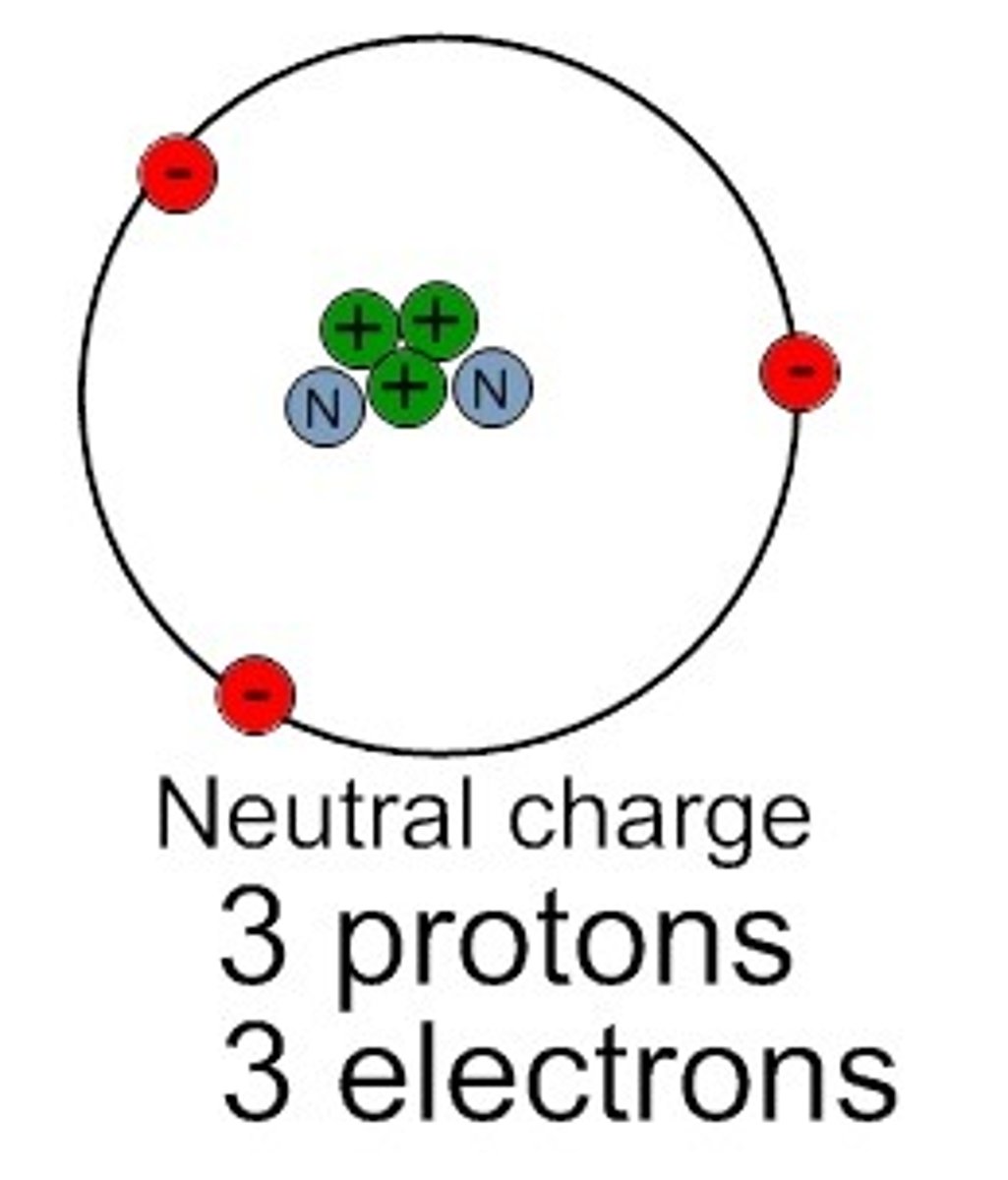
Neutral atom
Protons = Electrons
The number of protons and electrons are the SAME (equal) and the atom has NO CHARGE
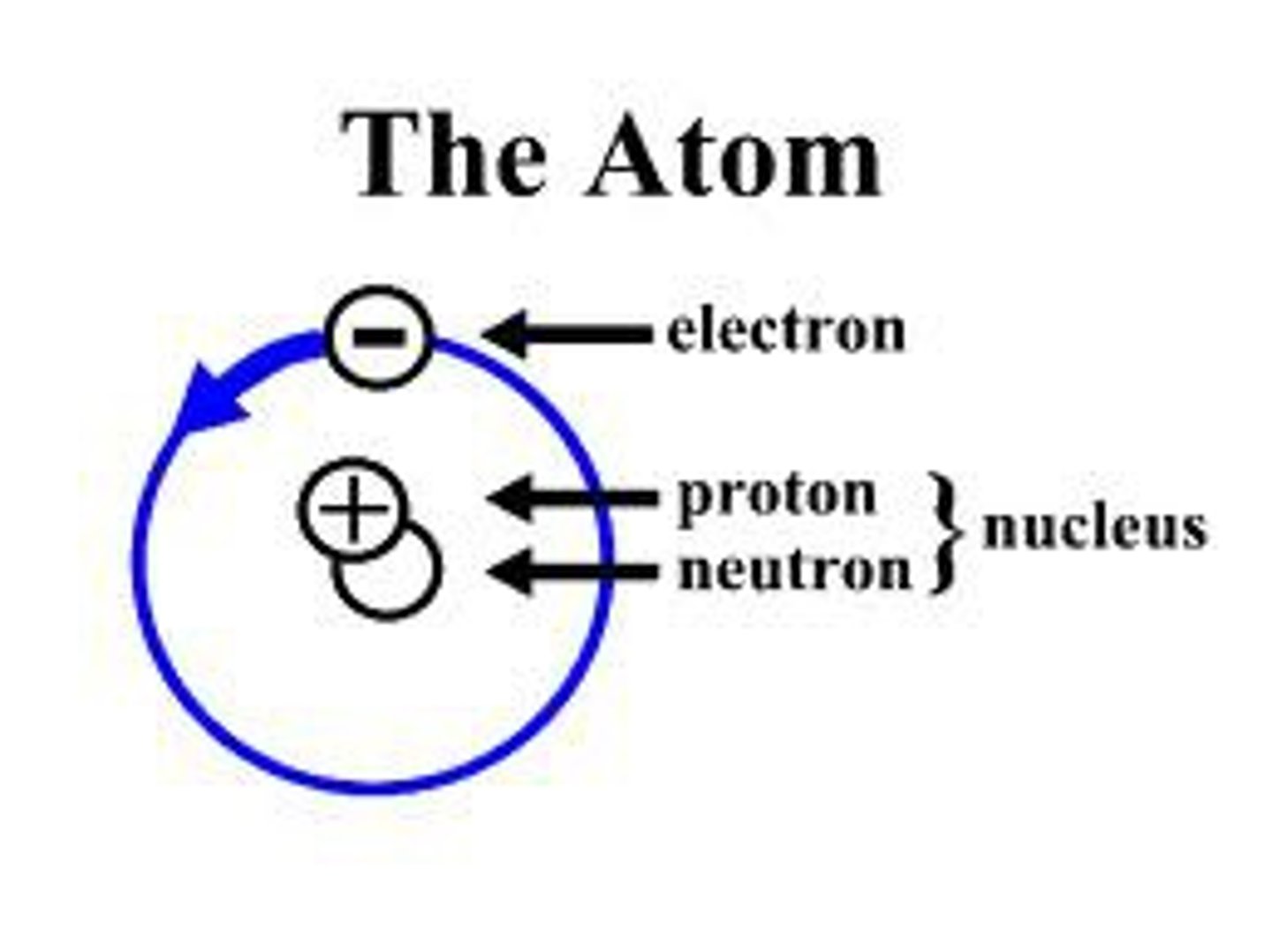
Atom
smallest part of matter that still acts like the element it is
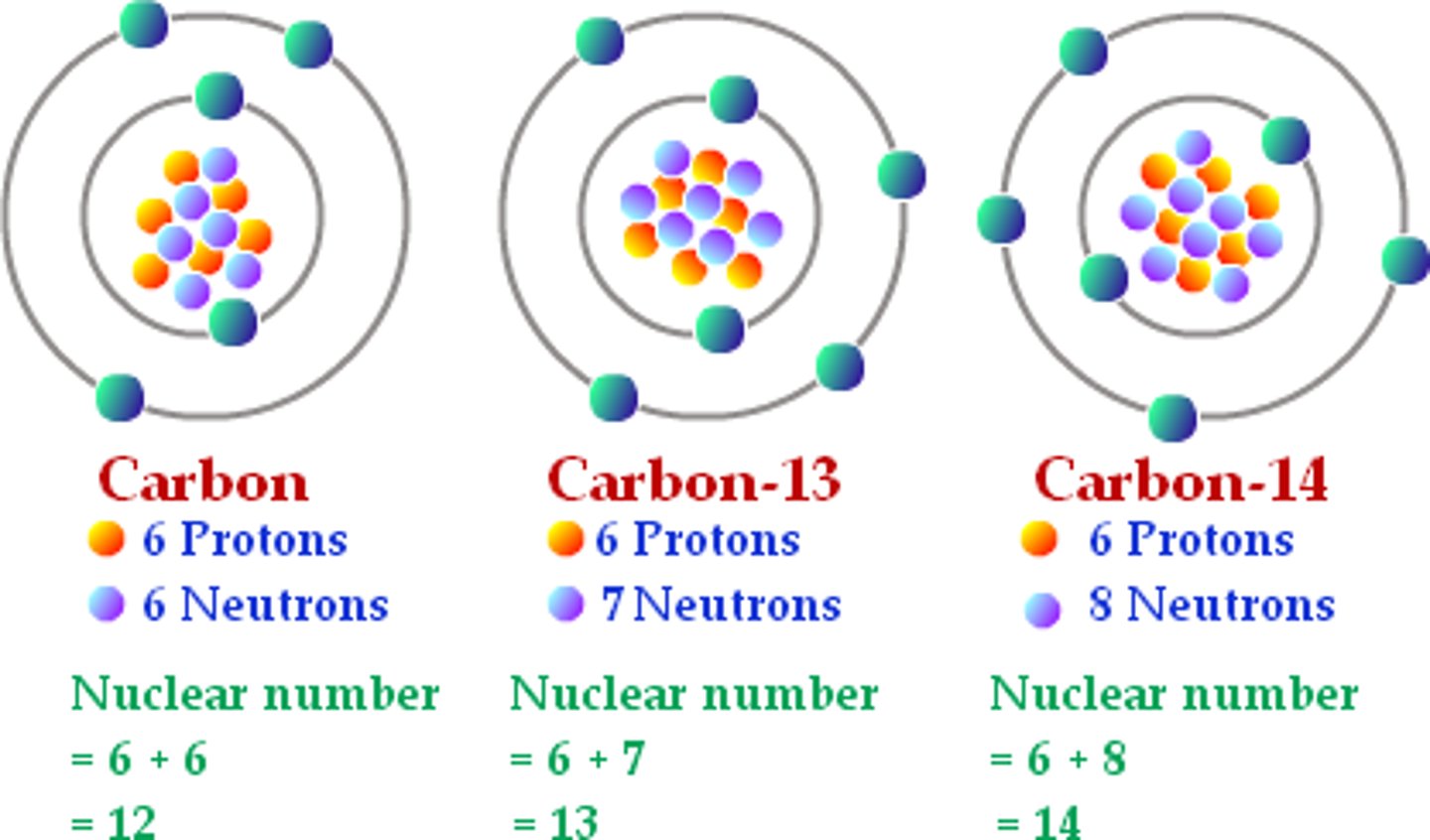
Isotope
Atoms of the same element that have different numbers of neutrons
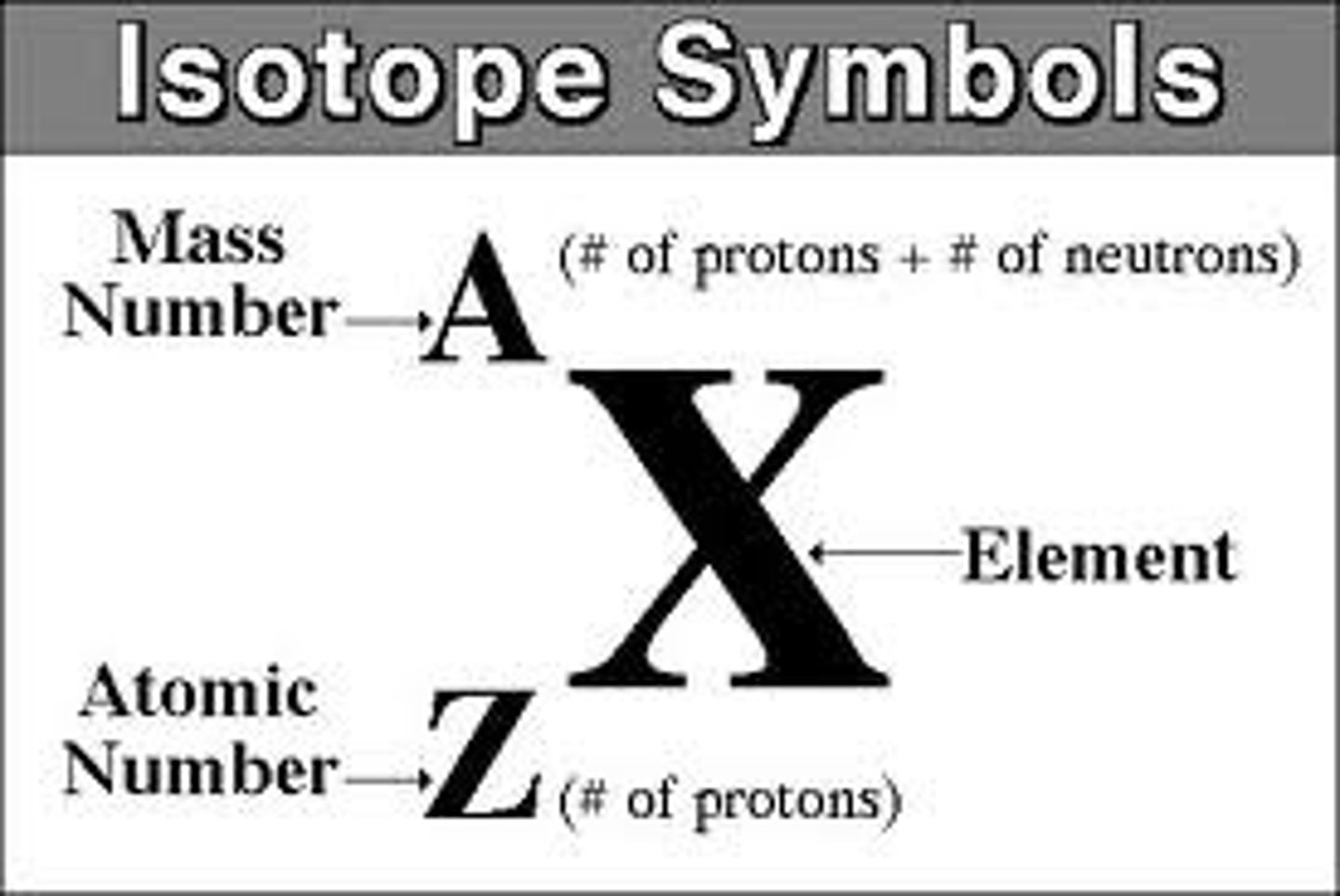
Isotope notation example
Isotopes of Carbon