Inorganic Unit 9
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
True or False: All Coordination complexes experience sigma bonding
True
Pi donor ligands typically…
have filled p orbitals
Pi acceptor ligands typically…
have unfilled p orbitals
pi donor ligands give ______ overlap
side-on
Pi donor ligands have ____ overlap
weaker
Pi acceptor ligands have _____ overlap
stronger
What type of ligand is OH- ?
sigma donor
pi donor
Phosphines are what type of ligands
sigma donors and pi acceptors
a low-lying P-R anti-bonding orbital can accept electrons in back-bonding
Define crystal field theory
An early idea based on metal in a crystal lattice. Treats metals in complexes as positive point charges in a field of negative point charges (the ligands)
Define ligand field theory
Theory that combines crystal field theory with MO theory to give a more complete understanding of coordination complex electronics rooted in bonding
What is a short-coming of crystal field theory?
Does not explain bonding or rationalize the impacts of neutral ligands
Do all ligands have a negative charge?
No
According to ligand field theory, lower _1__ orbitals are nonbonding ____(a,b,c)___
T2g
a. dxy
b. dxz
c. dyz
For an octahedral complex, ligand pi orbitals form blank, blank, blank, and blank
3 T1g and 3 T2u non-bonding SALCs
3 T1u SALCs which interact w/ metal p orbitals
3 T2g SALCs which have a crucial interaction w/ previously nonbonding metal d orbitals.
Rank the following ligands from weakest to strongest:
OH- I- H2O Cl- F- Br-
I- < Br- < Cl < OH- < F- < H2O
Rank the following ligands from weakest to strongest:
NH3 NCS-(N-bound) en CN- CO PR3 bipy
NCS-(N-bound) < NH3 < en < bipy < PR3 < CN- < CO
Rank the following ligands from weakest to strongest:
Br- NH3 H2O CN- F-
Br- F- H2O NH3 CN-
Weak field ligands have ___ 𐤃
low
Strong field ligands have ___ 𐤃
high
Strong field ligands are also pi ____
acceptors
Weak field ligands are also pi ____
donors
Most labile also means…
weakest field
Least labile also means…
strongest field
True or False: More labile (weak field) ligands can be displaced by stronger field ligands
True
Higher oxidation state = ____ splitting
larger
Larger metal size = ____ splitting
larger
3d metals < 4d metals <= 5d metals
-the smaller the metal, the smaller the orbital, the smaller the overlap
The color absorbed depends on 𐤃 and can be controlled by 1 & 2
Metal identity/oxidation state
Ligand field strength
stronger field ligands raise 𐤃 leading to absorption of higher energy colors like blue or violet
Draw the color wheel
Red: 650nm
Orange: 590nm
Yellow: 570nm
Green: 510nm
Blue: 475nm
Purple: 400nm
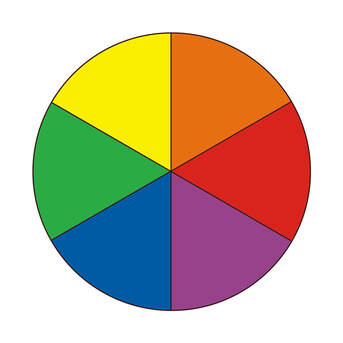
If a compound absorbs orange, you’ll see…
Blue
What are the three types of absorption?
MLCT (metal to ligand charge transfer)
LMCT (ligand to metal charge transfer)
LC (ligand centered)
To be stable, most metal complexes have ____ electrons total d and coordinating electrons
18
Square planar complexes are often stable at ___ electrons
16
This is to leave the highest d level empty
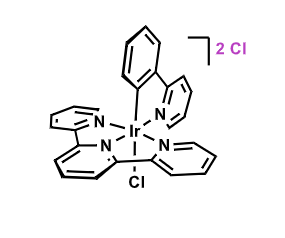
What color might you expect to see here?
Strong split suggests a big gap, so it would absorb purple and we would see yellow
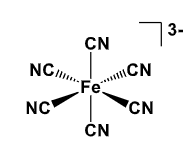
What is the electron count, and do you expect this to be stable?
17e-
it is unlikely to take on another ligand since its octahedral, and it is very unlikely to be reduced to gain stability
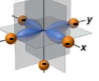
What orbital is this
dx²-y²
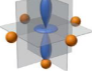
What orbital is this
dz²

What orbital is this
dxy
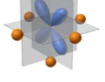
What orbital is this
dxz
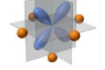
What orbital is this
dyz
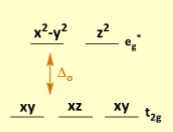
Which geometry does this represent
octahedral
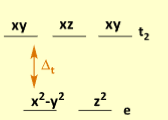
Which geometry does this represent
Tetrahedral
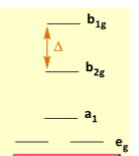
Which geometry does this represent
Square Planar
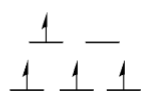
Is this high spin or low spin

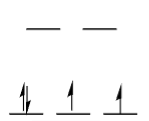
Is this high spin or low spin

What is MLCT?
Metal to Ligand Charge Transfer

What is LMCT?
Ligand to Metal Charge Transfer

What is LC?
Ligand-Centered

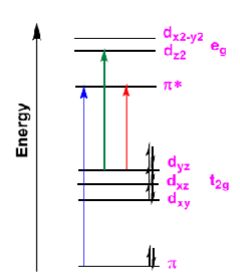
Identify which color is MLCT, LMCT, and LC
Blue: LC
Green: LMCT
Red: MLCT
What is the 18-electron rule?
To be stable, most metal complexes have 18 total d and coordinating electrons
• Similar to the octet rule for atoms but not derived from filling valence shell AOs