Exam 1 Organic Chemistry 2
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Aldehydes + Grignard
secondary OH
Ketones + Grignard
Tertiary OH
Formaldehyde + Grignard
Primary Alcohol
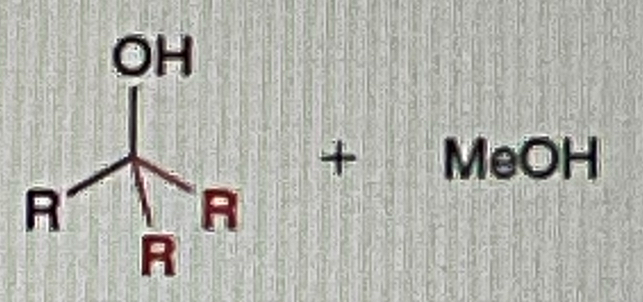
Ester/Acid Chlorides + Grignard
Tertiary OH
Nitriles (CN) + Grignard
Makes ketones
H2O will make
Just hydrogen
Whenever you the word “EXCESS” on the reagent
It’s mean use the reagent 2 TIMES
Carboxylic Acid /Aldehyde/Ketone/Ester + LiAlH4
Alcohols
OH-Ch2-Ch2-OH
Diol
What is a Diol
Protecting Group
What can transform Diol to carbonyl by ?
H+
Diol + H+
Carbonyl
Aldehyde + NaBH4, MeOH
Primary Alcohol
Aldehyde + H2/ Pt, Pd, Ni
Primary Alcohol
Aldehyde + LiAlH4/H2O
Primary Alcohol
Ketone + NaBH4, MeOH
Secondary Alcohol
Ketone + H2/ Pt,Pd,Ni
Secondary Alcohol
Ketone + LiAlH4/H2O
Secondary Alcohol
Carboxylic Acid + Excess LiAlH4/ H2O
Primary Alcohol
R-C=O-OMe + Excess LiAlH4/H2O
Primary Alcohol + MeOH
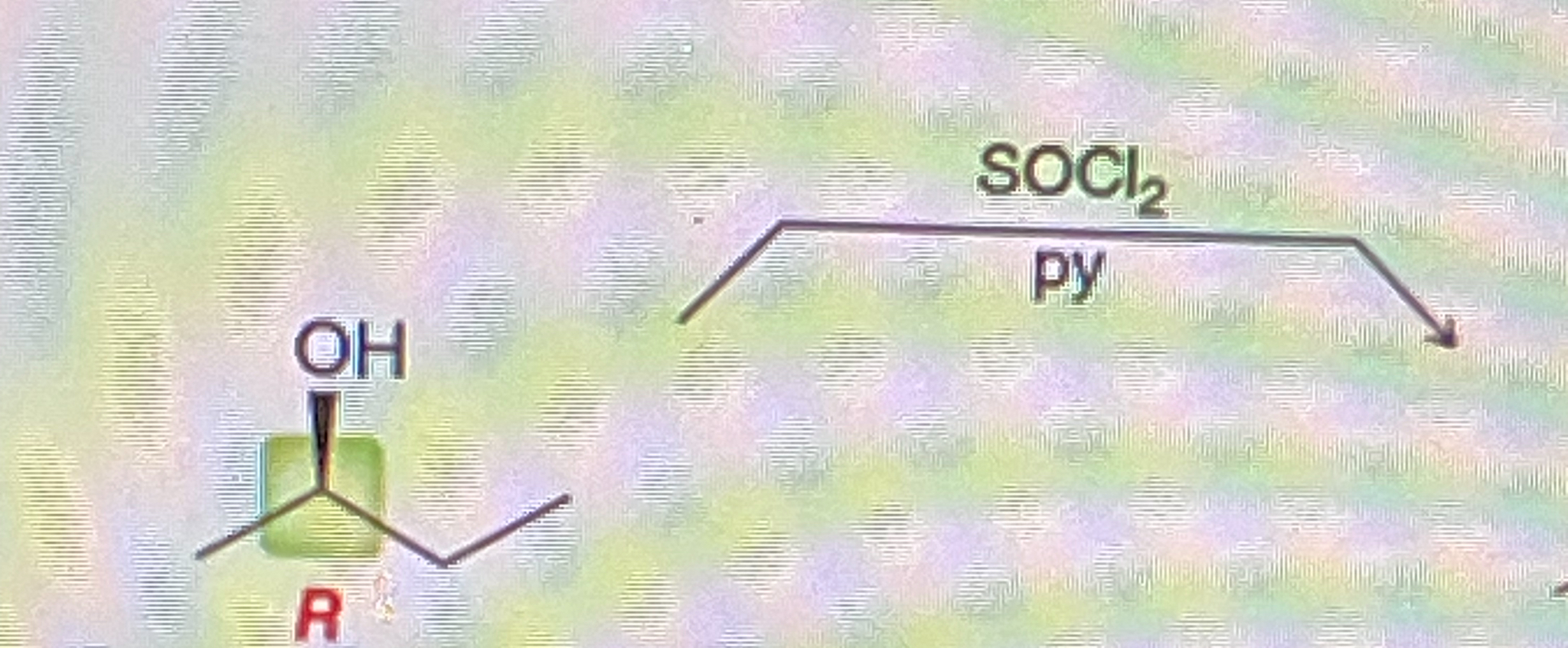
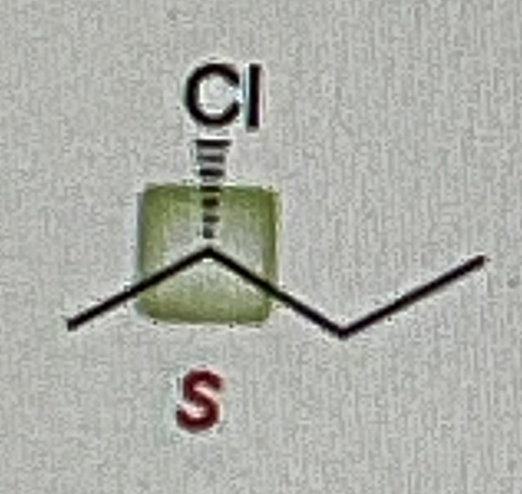
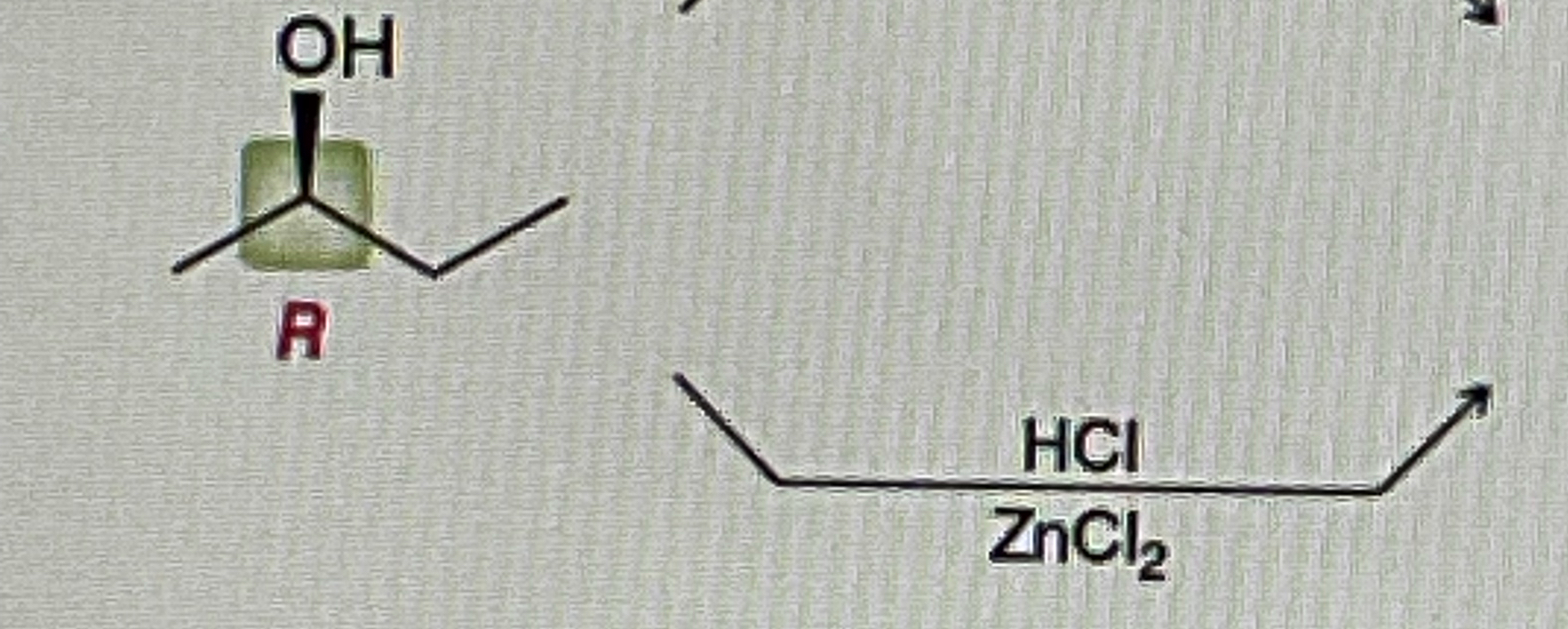
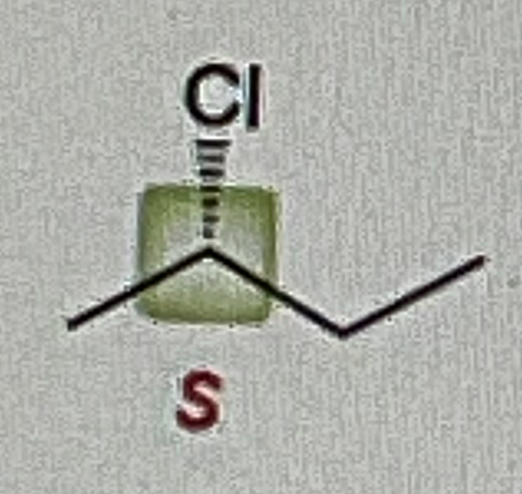
Secondary Alcohol + Na2Cr2O7/H2SO4, H2O
Ketone
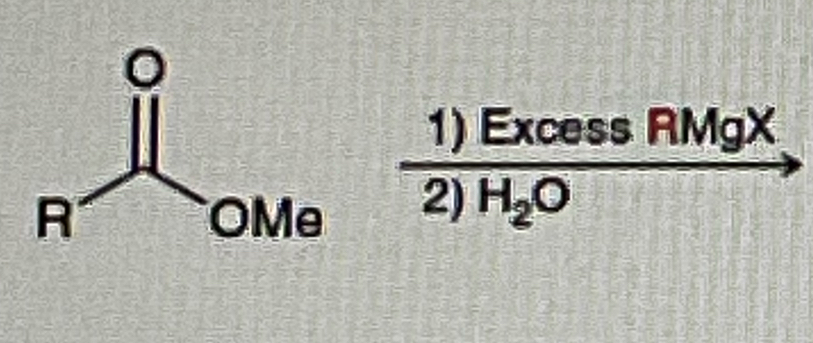
R-OH + TMSCl/ Et3N
R-O-TMS
R-O-TMS + TBAF
R-OH
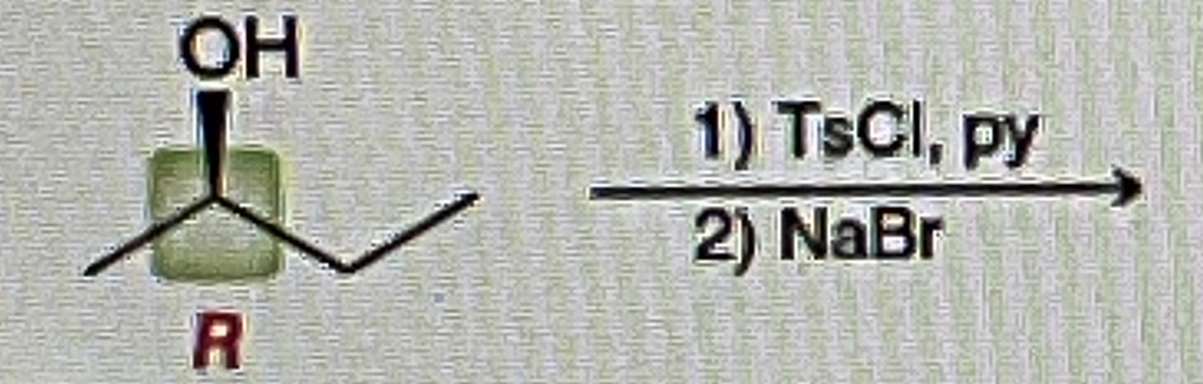
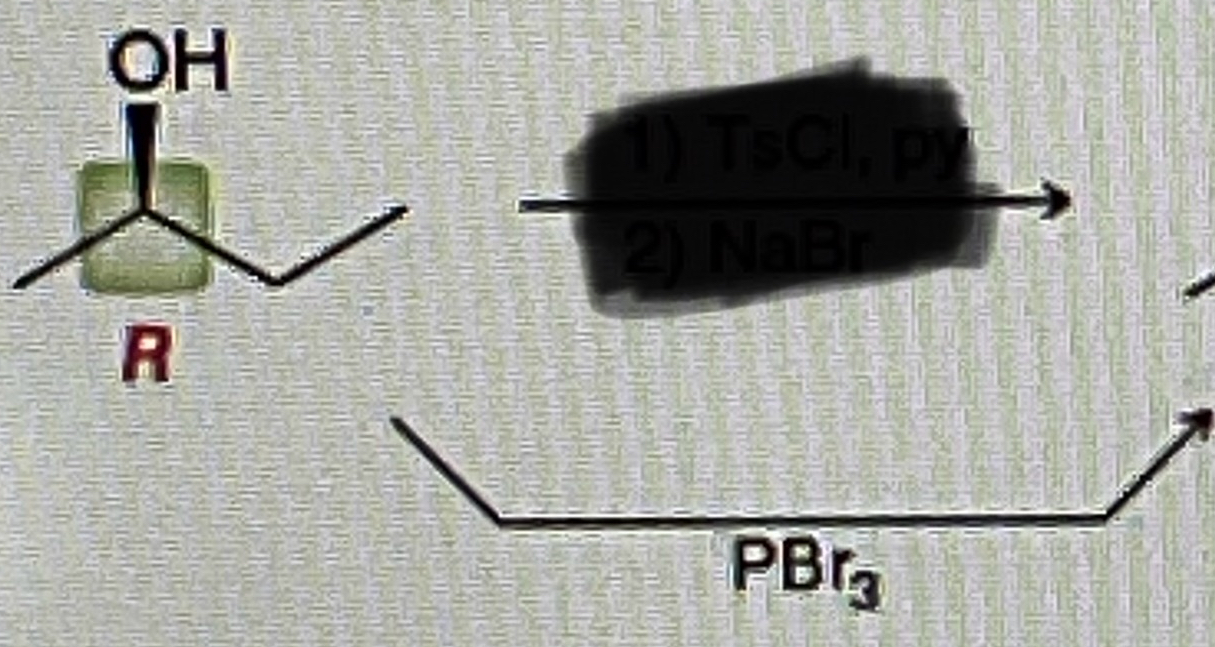
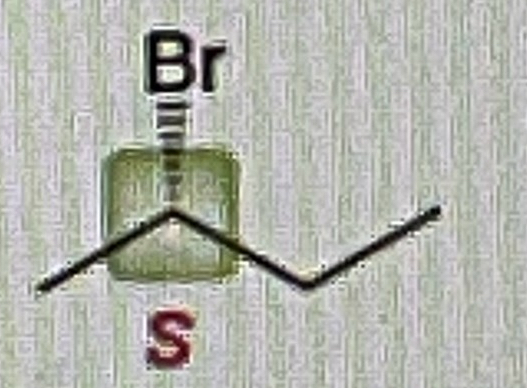
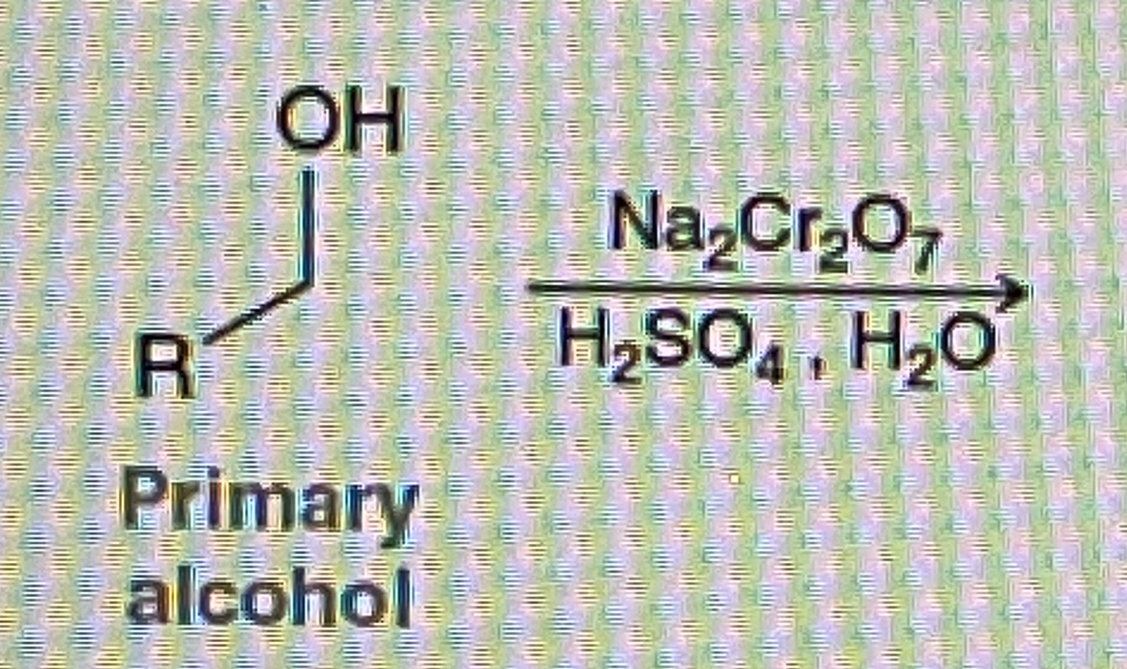
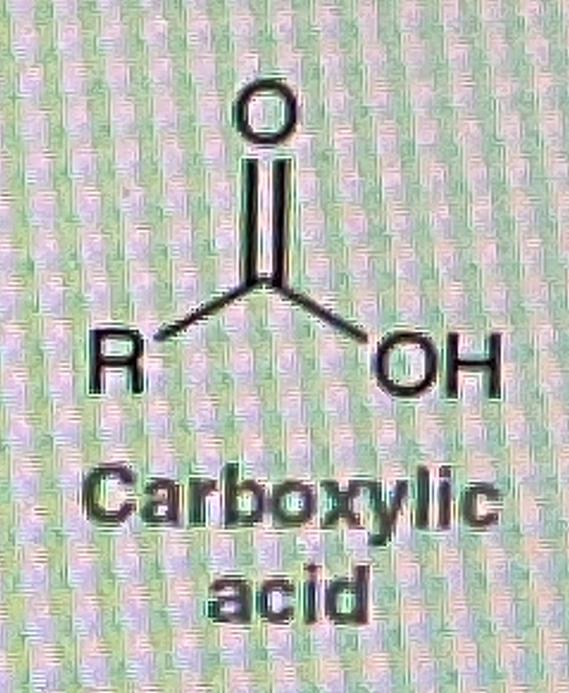
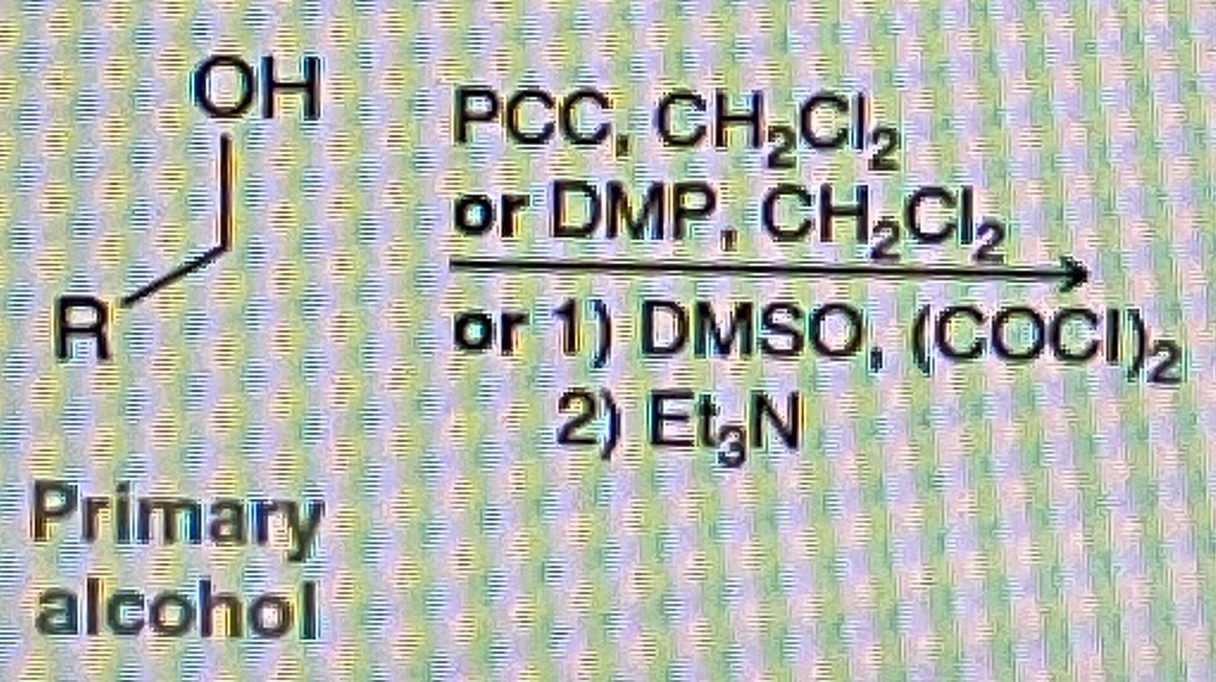
How do you make Carboxylic Acid from primary alcohol
Na2Cr2O7/H2SO4,H2O
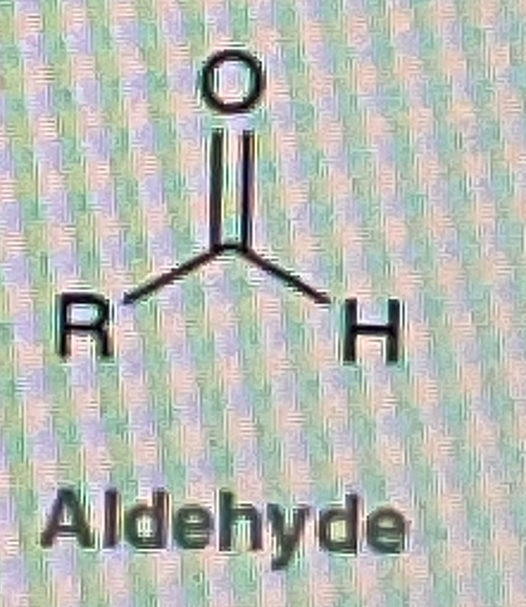
How do you make Aldehyde from a Primary Alcohol
PCC/Ch2Cl2
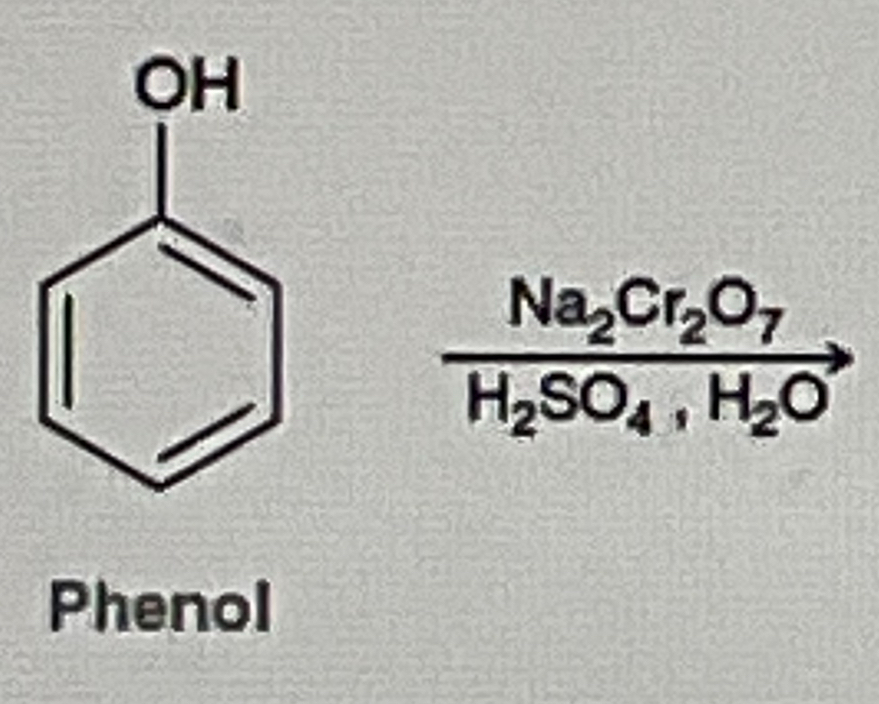
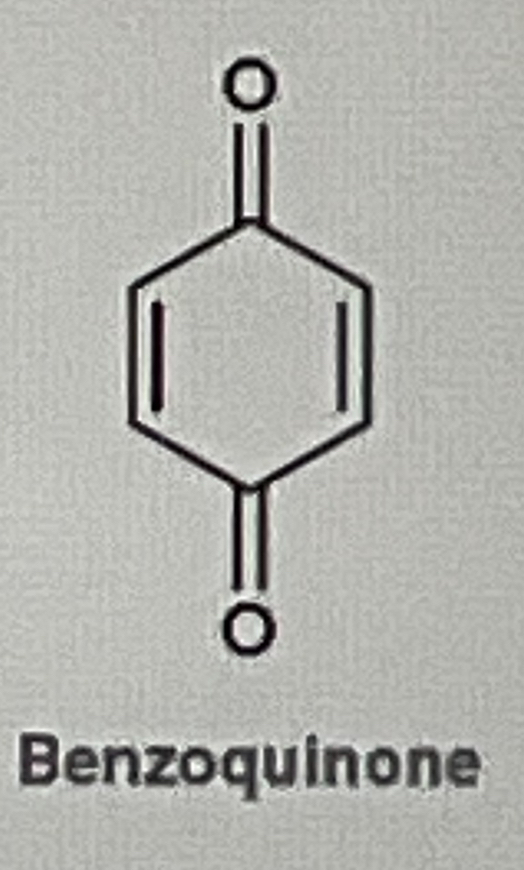
How do you make Benzoquinone from phenol
Na2Cr2O7/H2SO4,H2O
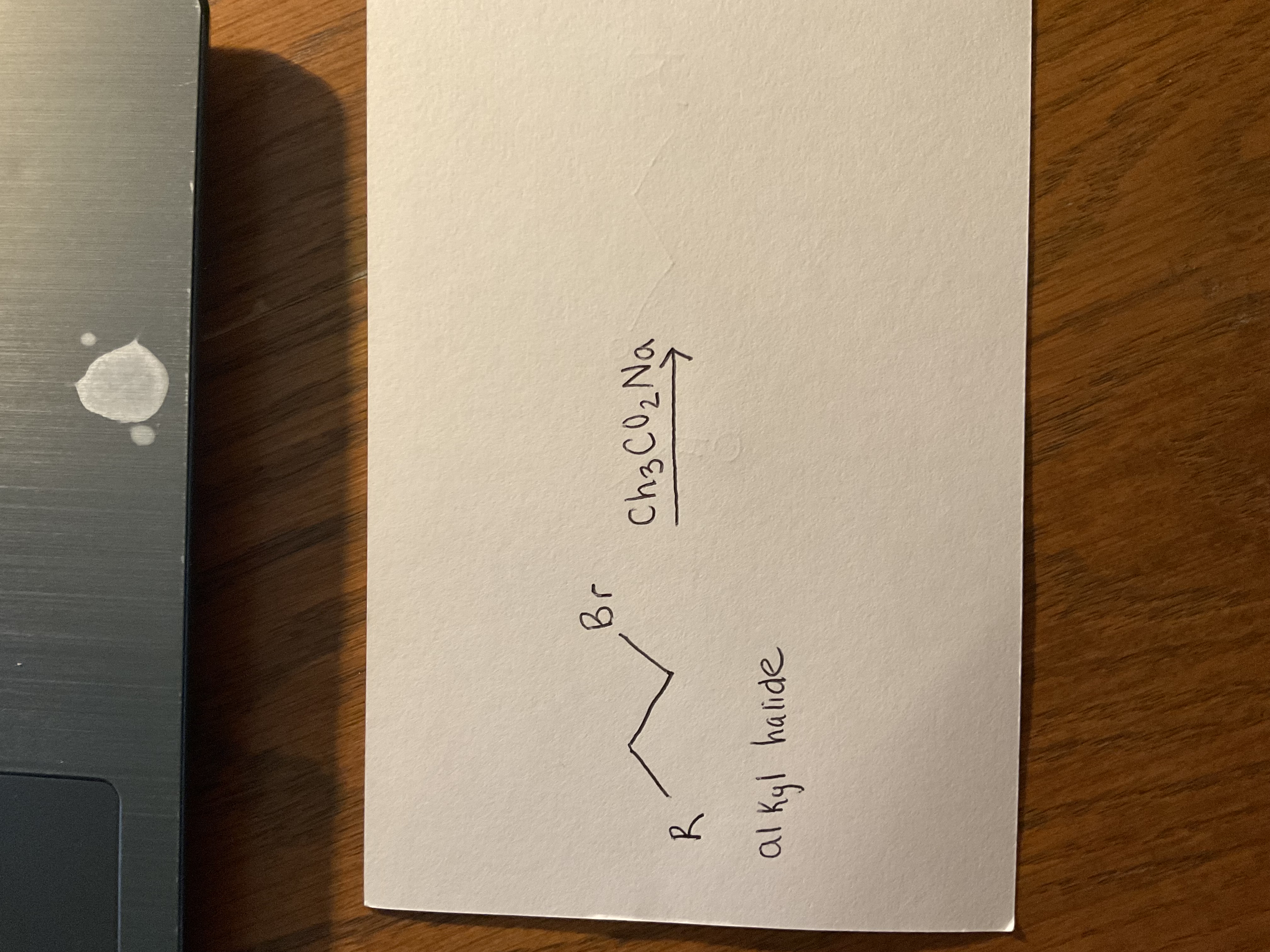
Alkyl halide + NaOH
Primary OH
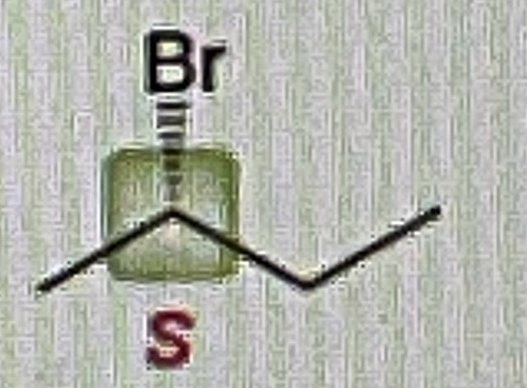
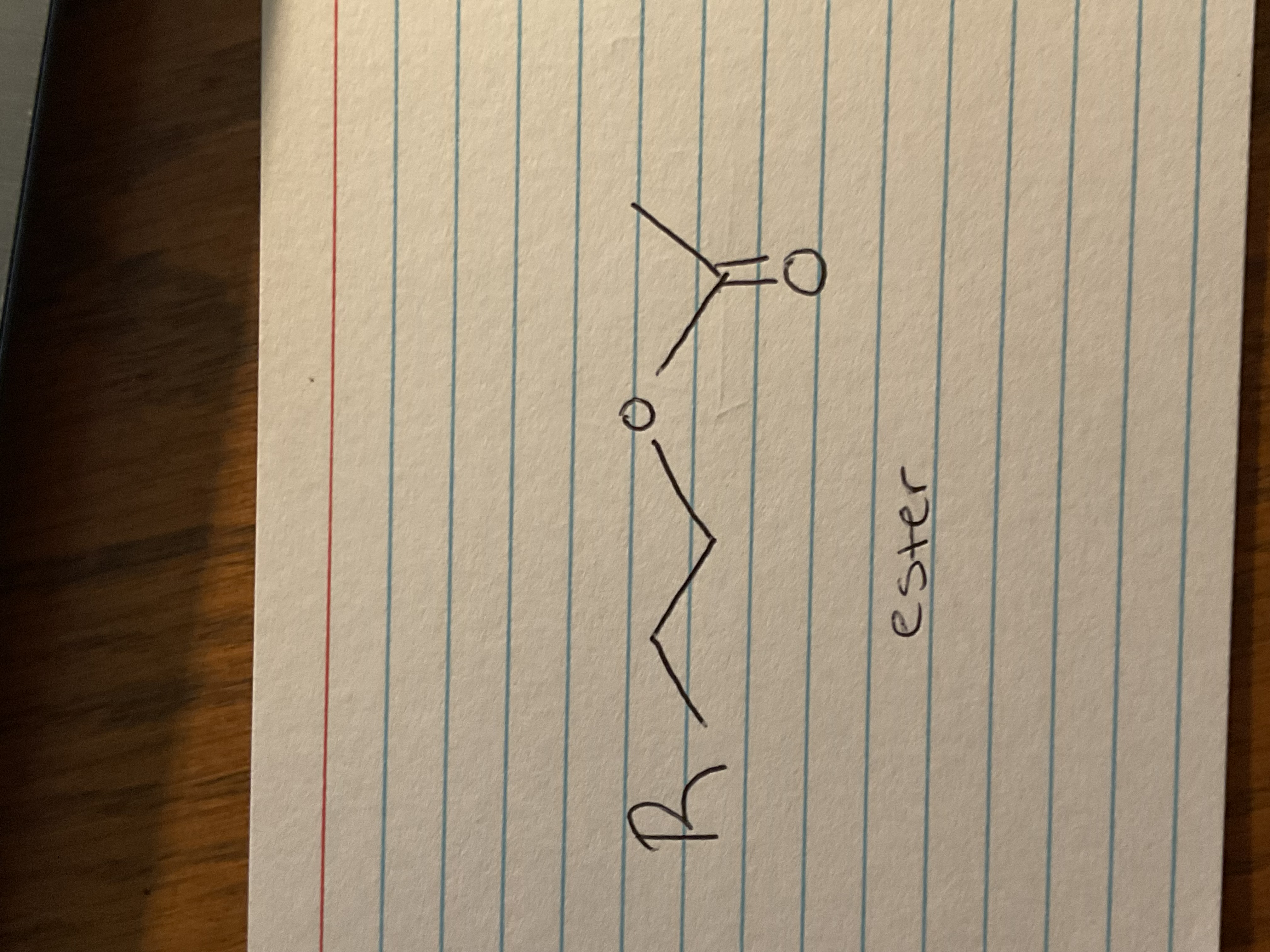
Primary Alcohol + TsCl, py
Primary OTS
Primary OTS + NaOH
Primary OH
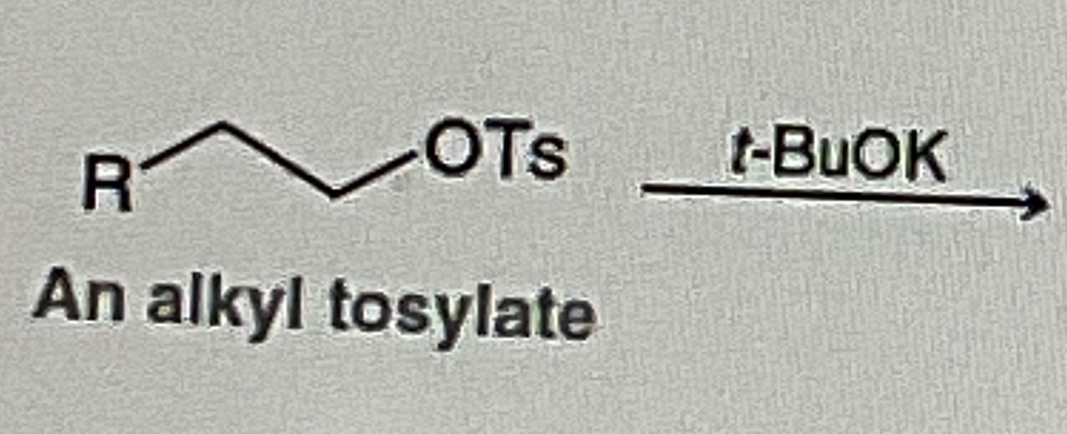
Alkyl Tosylate abbreviated
OTS
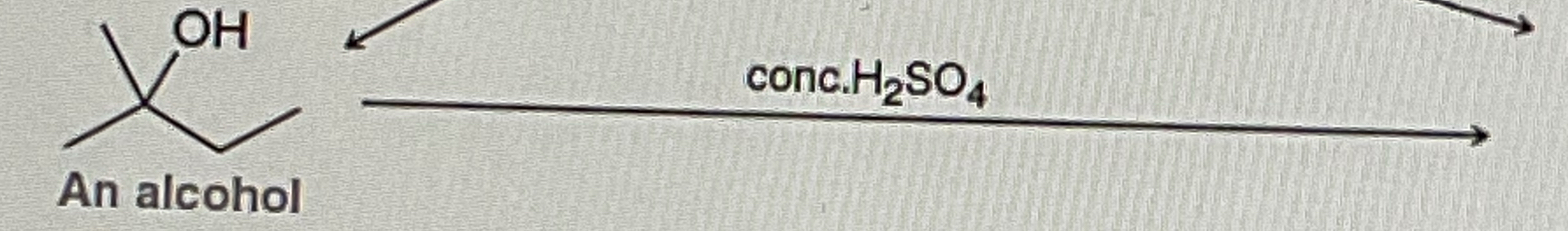
Tertiary Alcohol
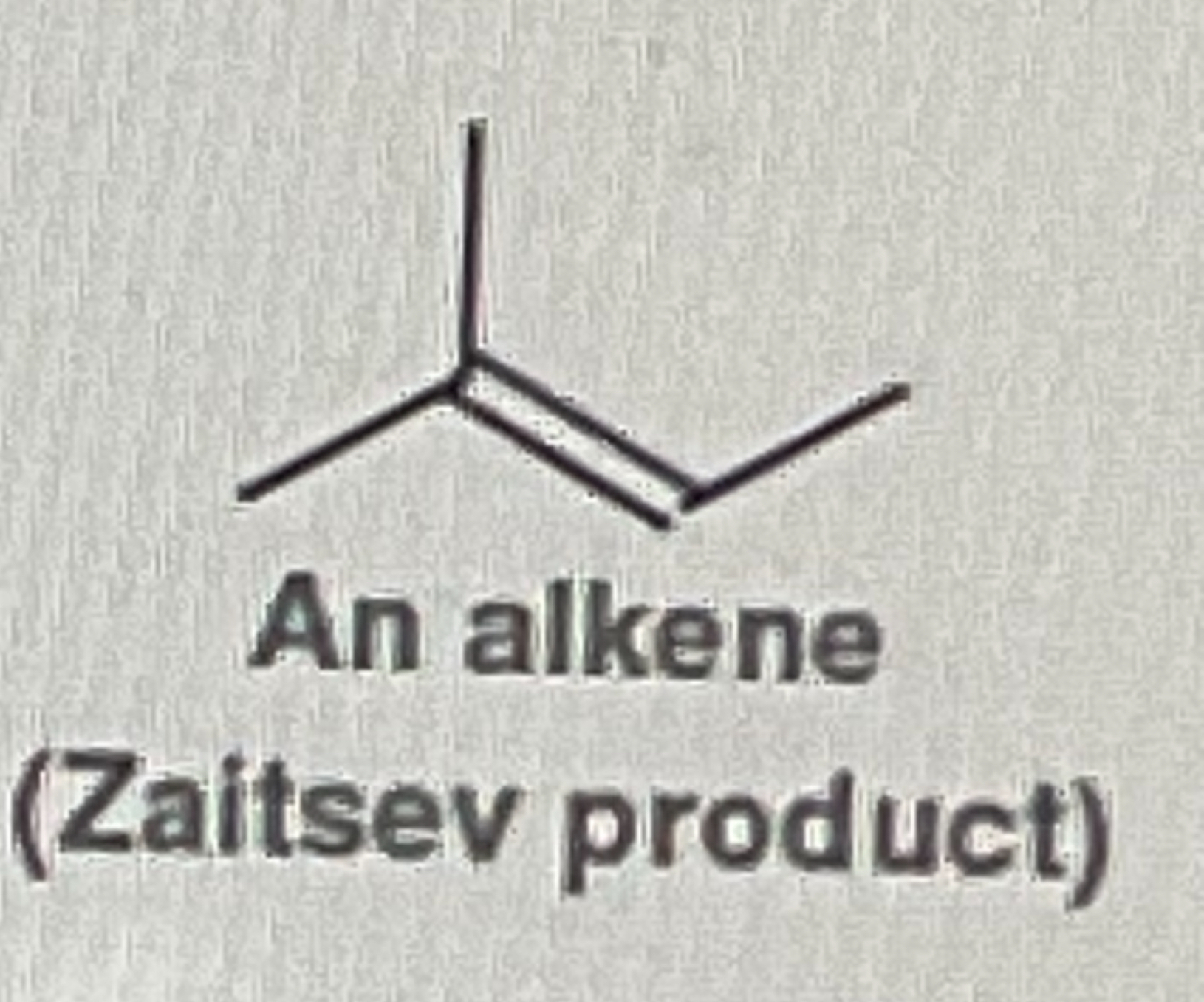
Tertiary Alcohol + Conc. H2SO4
OH will removed and form on Double Bond where the OH was

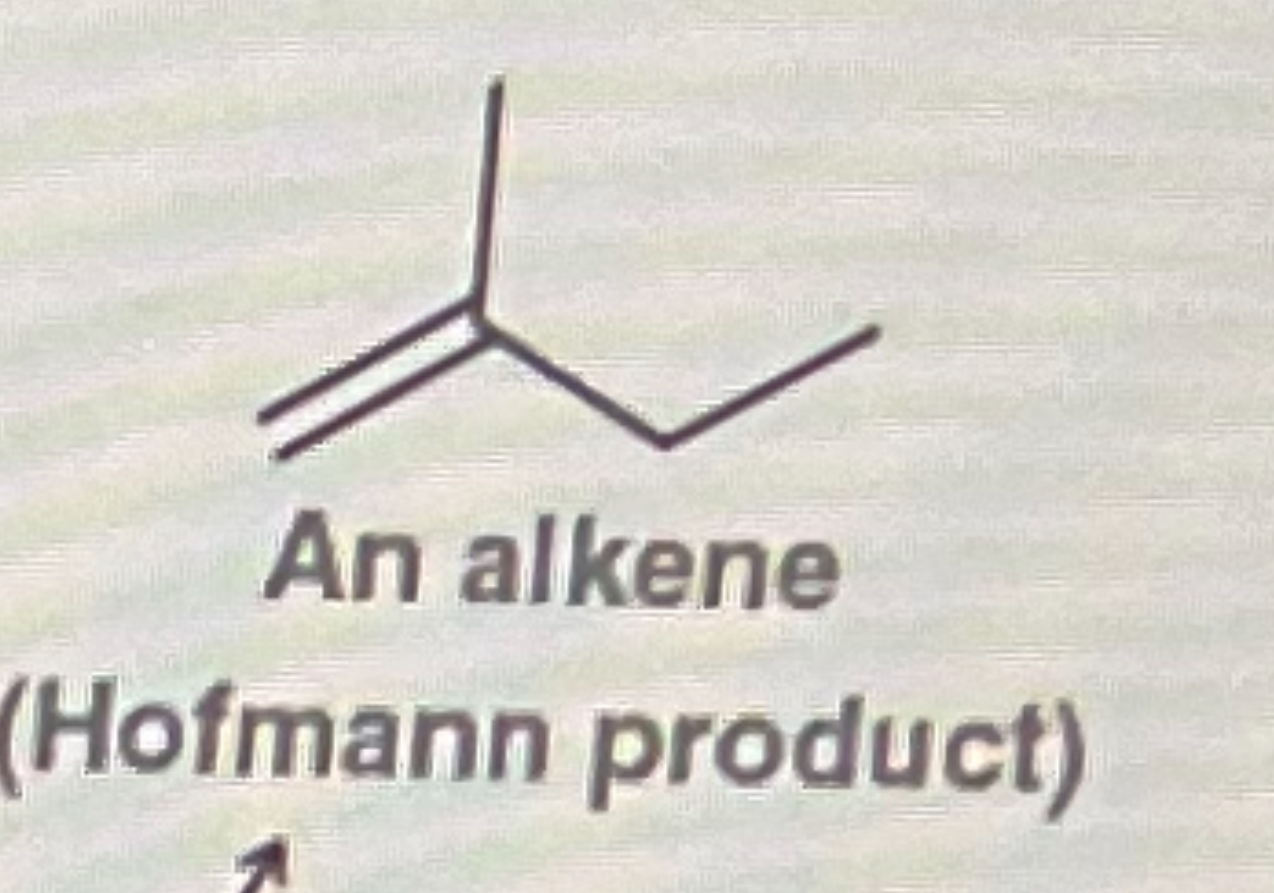
Tertiary Halogen + NaOH
Form double bond where the halogen was
Any tertiary halogen (alkyl halide) will form
Stable or unstable double bond
Tertiary OH + NaOH
Alkene where OH was removed
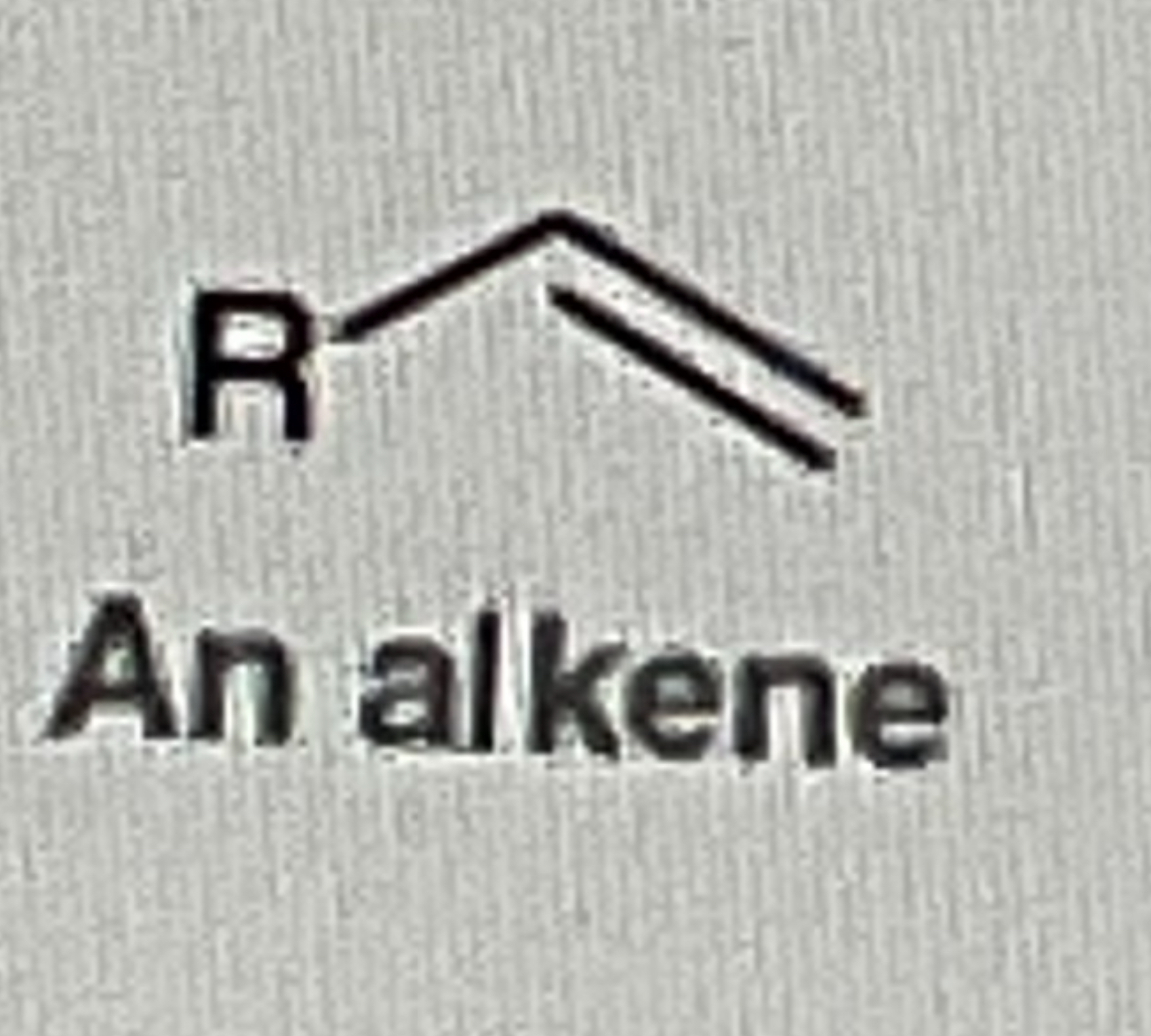
t-BuOK will form ?
Unstable Double Bond