Unit One Exam
1/163
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
164 Terms
What do I mean when I say that living systems are complex and highly organized?
Every cell is made up of tiny inorganic molecules that come together to form organic molecules that make up the structures and processes necessary for life. These interactions create a remarkable level of organization, from metabolite to organelle inside of a cell.
What is the smallest unit that makes up a cell?
an inorganic precursor
How does the cycle of energy transfer start in an ecosystem?
It starts with producers, primarily plants, that convert solar energy into chemical energy through photosynthesis.
The byproduct of photosynthesizers is the substrate which consumers use to gain energy. What is this?
Carbon from carbon dioxide which forms sugars which are eaten by the consumer.
What defines a system to be living?
That the system can self-replicate.
What are the four elements and the acronym to remember them by that make up nearly all of a living organism?
Carbon, Hydrogen, Oxygen, and Nitrogen; CHON
Why is it that carbon, hydrogen, oxygen, and nitrogen make up most of living organisms?
The electron configuration of the molecules leads to highly favorable reactions between the four of them. These reaction make highly stable covalent bonds.
What does it mean for a biomolecule to be a directional polymer?
It refers to the idea of there being a correct direction for the molecule to be read or operate from. This is true for amino acids especially which have a front terminus that is always a nitrogen group and an end terminus which is always a Carbon.
What is the directionality of DNA
The front (5’) terminus is a phosphate group and the end (3’) terminus is a hydroxyl group.
If we say that a biological macromolecule is informational, what do we mean?
It means that the macromolecule contains coded information needed for biological processes, such as genetic information in DNA or instructions for protein synthesis.
Biological macromolecules are three dimensional. They are not a flat object; therefore, what is the most common type of model to express macromolecules in biochemistry?
Specifically for proteins, we express the structural motifs using ribbon and space-filling models.
Weak intermolecular interactions maintain biological structures, what is the first one discussed?
Van der Waals forces which are weak attractions between molecules that occur when they are in close proximity due to induced dipoles between atoms. These forces play a crucial role in stabilizing macromolecular structures and interactions.
Weak intermolecular interactions maintain biological structures, what is the second one discussed?
Hydrogen bonds, which are attractions between a hydrogen atom covalently bonded to an electronegative atom and another electronegative atom, contributing to the stability of protein and nucleic acid structures.
Weak intermolecular interactions maintain biological structures, what is the third one discussed?
Ionic bonds, which are attractions between positively and negatively charged ions, playing a significant role in the stability and interactions of macromolecules.
Weak intermolecular interactions maintain biological structures, what is the fourth one discussed?
Hydrophobic interactions, which occur when nonpolar molecules aggregate in aqueous environments to minimize their exposure to water, thereby influencing the folding and stability of proteins.
What is lock and key biomolecular recognition?
A model suggesting that enzymes and substrates fit together precisely like a key in a lock, allowing for specific binding and catalysis.
What is induced fit biomolecular recognition?
A model that suggests that a substrate attaches to an enzyme in a way where the shape of the enzyme is altered to accomodate the substrate. This allows the enzyme to operate with multiple substrate types.
True or False: H2O is only a hydrogen bond donor.
False, H2O can act as both a hydrogen bond donor and acceptor.
Since the number of H-bonds that a water molecule can form is balanced, what is the meaning of it?
This means that water has a unique ability to stabilize its molecular structure, allowing for properties like cohesion, adhesion, and high surface tension. Also menaing that water can interact with up to four other molecules.
What three atoms take part in H-bonding?
Fluorine, Nitrogen, and Oxygen
Why is the density of solid water lower than the density of liquid water?
The density of solid water (ice) is lower than that of liquid water due to the hydrogen bonds forming a crystalline structure that spaces the molecules further apart, making ice less dense.
How many H-bonds are in liquid water per atom?
About 3.4 H-bonds
How many H-bonds are in solid water per atom?
Exactly 4 H-bonds
Other than density what else is unique about water?
Water has a high specific heat capacity, meaning it can absorb a lot of heat without a significant increase in temperature.
What is the Gibbs Free Energy Equation?
∆G = ∆H - T∆S
Is negative or positive ∆G spontaneous?
Negative ∆G indicates a spontaneous process.
If you want a spontaneous reaction what must the value of ∆S be?
The value of ∆S must be positive to favor spontaneity, particularly when ∆H is also negative.
If you want a spontaneous reaction what must the value of ∆H be?
The value of ∆H must be negative or zero to favor spontaneity, especially when ∆S is positive.
If a reaction is nonspontaneous, what does this mean for the reverse of the reaction?
It is spontaneous
What is the first step of dissolving a compound in water?
Breaking the water H-bonds (∆H1)
What is the second step of dissolving a compound in water?
Breaking the solute interactions (i.e. Ionic interactions of a salt) (∆H2)
What is the third step of dissolving a compound in water?
Forming a new water-solute interaction (∆H3)
What types of reactions are present in the third step of dissolving a compound in water
London Dispersion (Vander Waals), H-Bond, Dipole-Dipole
Why is the enthalpy of the third step of dissolving a compound in water the opposite sign of the first two steps?
Because the formation of new interactions releases energy, while breaking existing interactions requires energy input.
What is a Amphipathic compound?
A compound that has both hydrophilic and hydrophobic properties, allowing it to interact with both water and lipids. i.e. Polar and Nonpolar regions are present.
True or False: Polar compounds dissolve in water?
True, because polar compounds can form hydrogen bonds with water, allowing them to dissolve readily.
What type of interaction dominates the system with polar compounds in water?
Dipole-Dipole interactions, unless there is H-bonding present.
Polar compounds dissolved in water are very dynamic. What does this mean?
It means that H-bonds are continuously formed and broke leaking to a ∆S of about zero.
Is there a release or consumption of energy when ion-dipole interactions occur with charged solutes?
There is a release of energy when ion-dipole interactions occur, as the attractive forces between ions and polar solvent molecules stabilize the system.
Why is there an increase in entropy when crystalline substances dissolve?
Dissolving crystalline substances increases entropy because the ordered structure of the solid breaks apart, leading to a more disordered mixture of solute and solvent.
Since Ion-Dipole bonds are continuously broken and formed, what does this mean?
It indicates that the entropy of the system is going to remain unchanged.
Even nonpolar compounds have some degree of polarity, which leads to what in a mixture with water?
the formation of dispersive interactions.
When in a nonpolar compound are compensating interactions formed with the solute after H-bonds between water molecules are broken?
No, instead the enthalpy of the system increases as H-bonds are broken. This means that although enthalpy increases, it does not lead to favorable mixing with water.
The aggregation of non-polar molecules to minimize surface exposed to solvent causes what?
A weakly positive ∆S
Since water molecules actively try to cage non-polar structures, what happens?
The water molecules are constrained and entropy is decreased
What is a structure that amphipathic compounds form?
Micelles which are spheres with hydrophilic heads facing outwards and hydrophobic tails facing inwards. This minimizes the surface area exposed to water and ensures the stability of the structure.
Why do non-polar and amphipathic structures for micelles?
It is a way to maximize thermodynamic stability by maximizing entropy of water.
What is a Brønsted Lowry Acid
A proton donor
What is the equation for the ionization of water?
2 H2O ←→ H3O+ + OH-
Water is a bulk solution which leads to a phenomenon called proton relay. What is proton relay?
Proton relay is the process by which protons are rapidly transferred between water molecules, facilitating efficient acid-base reactions. AKA FAST due to number of molecules and collisions occuring.
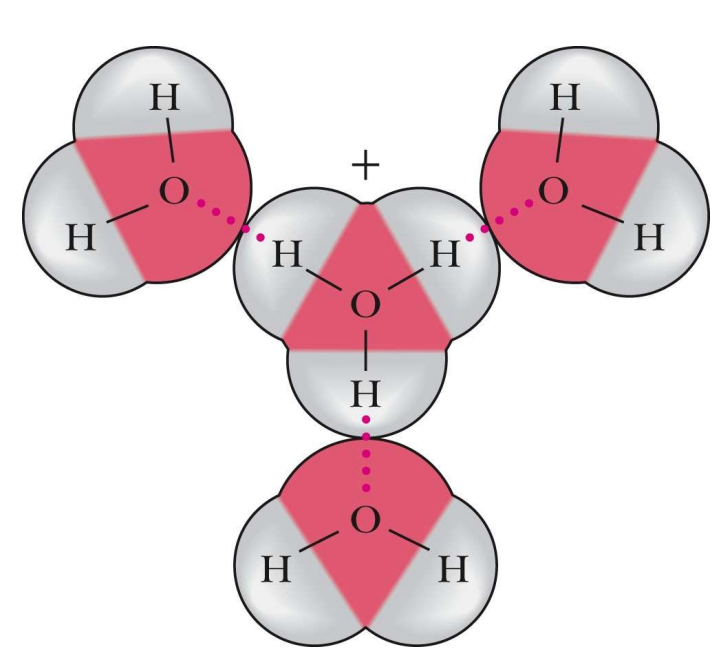
Why do water molecules come into a lattice structure at all if there is proton relay occurring between atoms?
Due to stable hydrogen bonds that form randomly from a collision, a lattice can be constructed.

What is the density of water?
1 kg/L or 1000 g/L
What is the molecular weight of water?
18.0 g/mol
What is the Molarity of water at 25ºC?
55.5 M
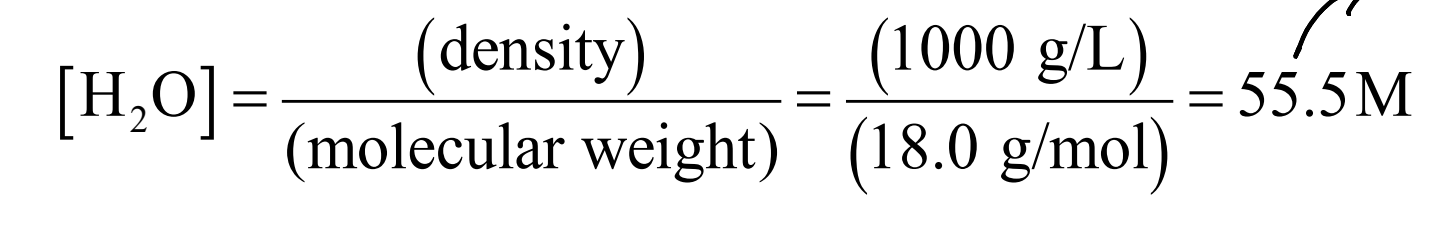
True or False: Due to the large difference in concentration between water molecules and solute molecules, the Molarity of water is considered a constant.
True, typically the Molarity of water is 3 magnitudes greater than the Molarity of solutes.
What is the equation of the equilibrium constant of water?
Keq = ([H+][OH-]) ÷ [H2O]
What is the value of the equilibirum constant of the ion product of water?
1.8 × 10-16
What is the value of the product of the concentrations of the hydronium and hydroxide ions in a solution of water?
1.0 × 10-14
What does pH + pOH equal?
14
What is Log(10)?
1
What is log 1?
0
What is 10logx ?
x
What is log(xy)?
Log(x) +Log(y)
What is Log (x/y)
Log (x) - Log (y)
What is Log (xy)?
ylog(x)
How are [H+] and [OH-] related?
They are inversely proportional.
What is the equation of a weak acid?
HA ←→ H+ + A-
What is the equilibrium constant of a weak acid?
Ka = ([H+][OH-]) ÷ [HA]
What is the log of Ka equal to?
log (([H+][OH-]) ÷ [HA])
What is the Henderson-Hasselbalch Equation?
pH = pKa + log ([A-] / [HA])
What is so special about the Henderson-Hasselbalch equation?
If you know the pKa for an acid and its conjugate base, you can solve for the pH at certain concentrations of acid and base; vice-versa.
If pH = pKa then …
The concentrations of the weak acid and conjugate base are equivalent.
What is the definition of a buffer?
A solution that resist changes in pH but only around pKa.
What does the relationship look like when we add hydroxide to a solution of acetic acid? (CH3COOH + OH- → CH3COO- + H2O)
.
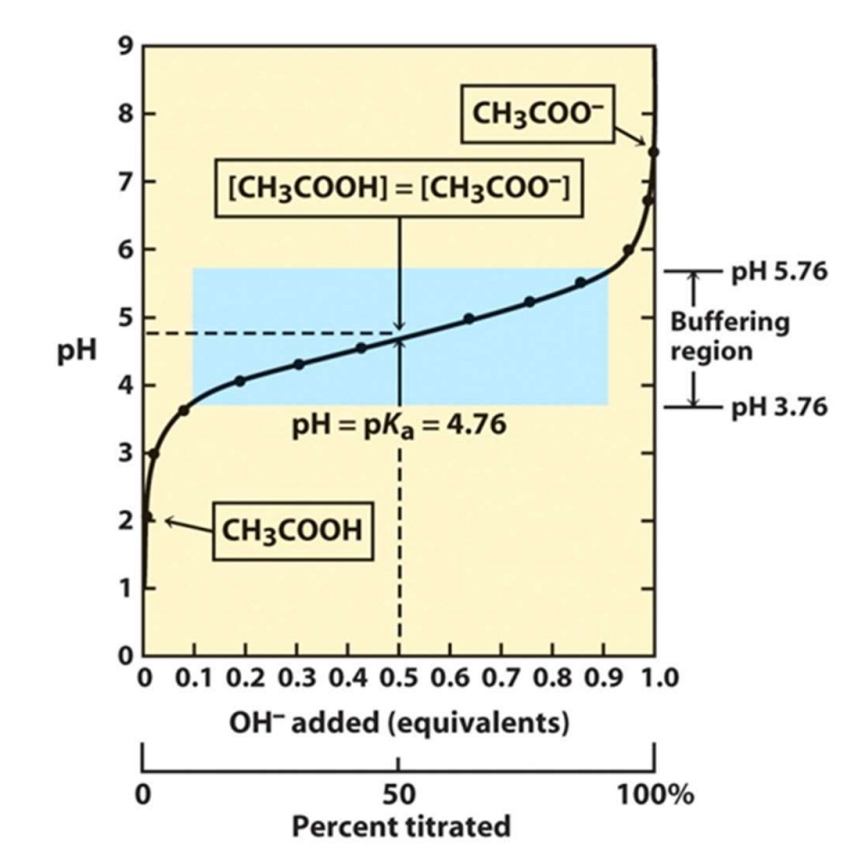
Where does pKa lie in a buffered solution?
In the middle of the buffer region (Also known as equilibrium point)
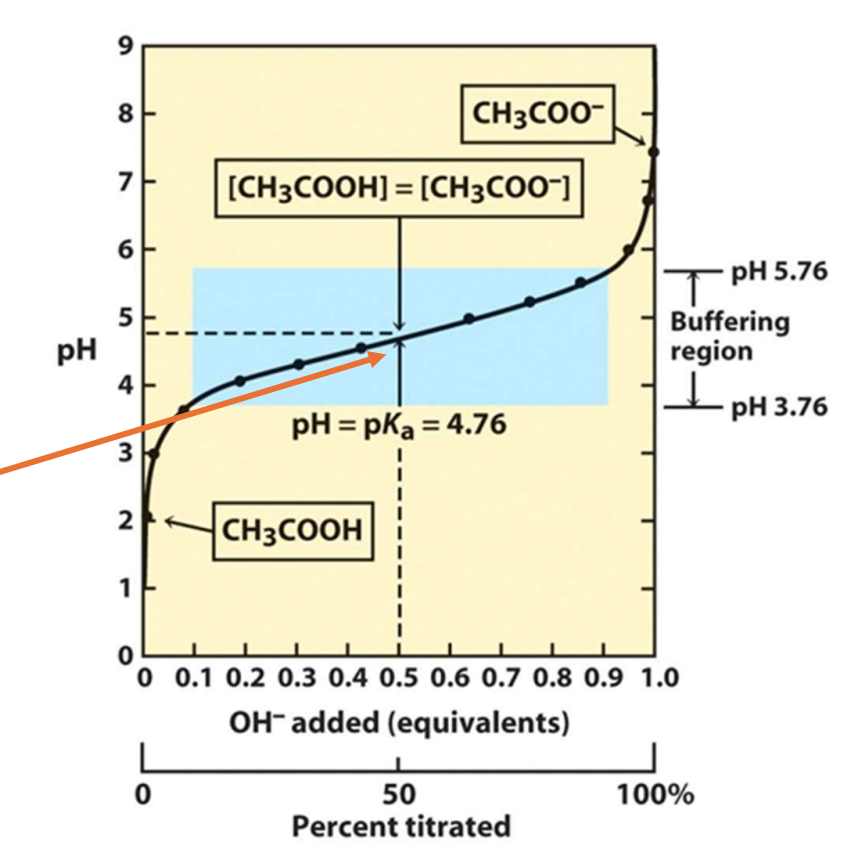
For a triprotic acid how many pKa values can you expect?
Three
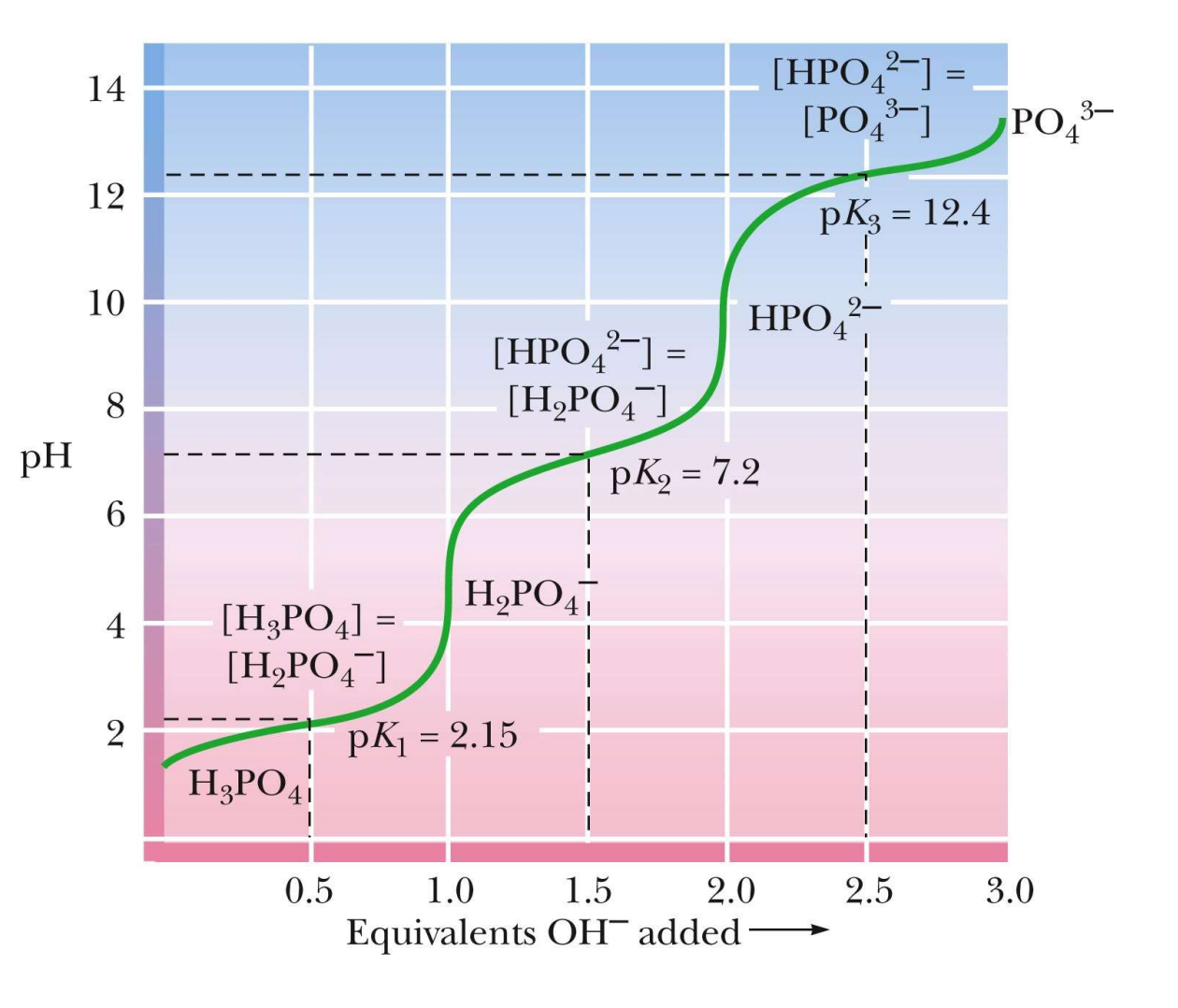
What is the standard range of a buffer region?
Typically 1 pH unit above and below the pKa value.
What is a condensation reaction?
A water forming reaction. A chemical reaction where two molecules combine to form one larger molecule, typically releasing water as a byproduct.
What is a hydrolysis reaction?
A reaction where water is broken down. In a chemical reaction, a larger molecule is split into smaller molecules by the addition of water.
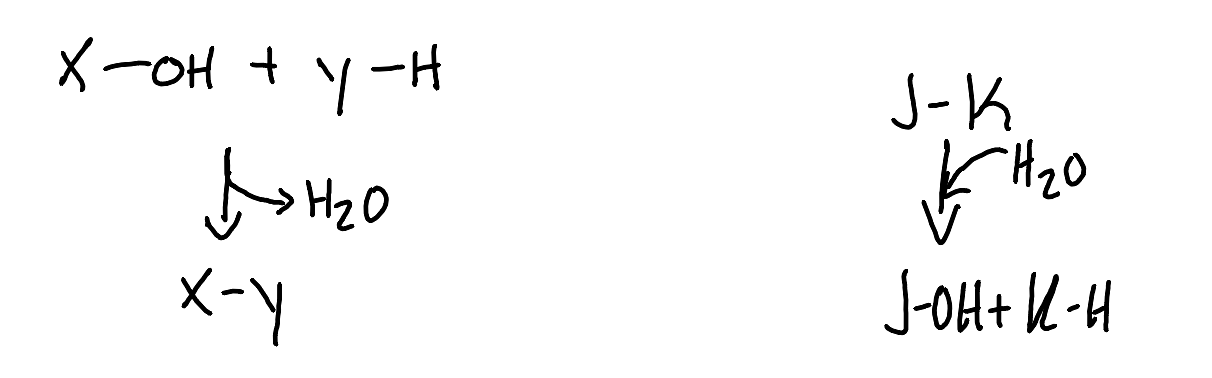
If X/Y/J/K concentrations are µM or mM, which type of reaction will be thermodynamically favorable in water?
The hydrolysis reaction which includes J & K because of the presence of such a vast amount of water molecules compared to the analytes.
How many amino acids are there?
23 amino acids that are proteinogenic amino acids (common amino acids, coded amino acids)
What does proteinogenic mean?
An amino acid that is incorporated during translation (protein biosynthesis process)
What is the basic structure of an amino acid?
Each amino acid has the same overall features: alpha carbon (central C), amino group (NH3+), carboxyl group (-COOH), H-atom, aand an R-group.
In an amino acid what parts of the amino acid bind to adjacent amino acids?
The amino group and carboxyl group
All amino acids other than glycine are chiral, why is glycine not?
Glycine’s R-Group is a H-atom which means that the molecule has 2.
What is the pKa of the carboxyl group of an amino acid?
2.0
What is the pKa of the amino group of an amino acid?
9.6
Why does glycine have a neutral charge at pH 7?
This is due to the amino group being protonated and the carboxyl group being deprotonated, resulting in a net charge of zero.
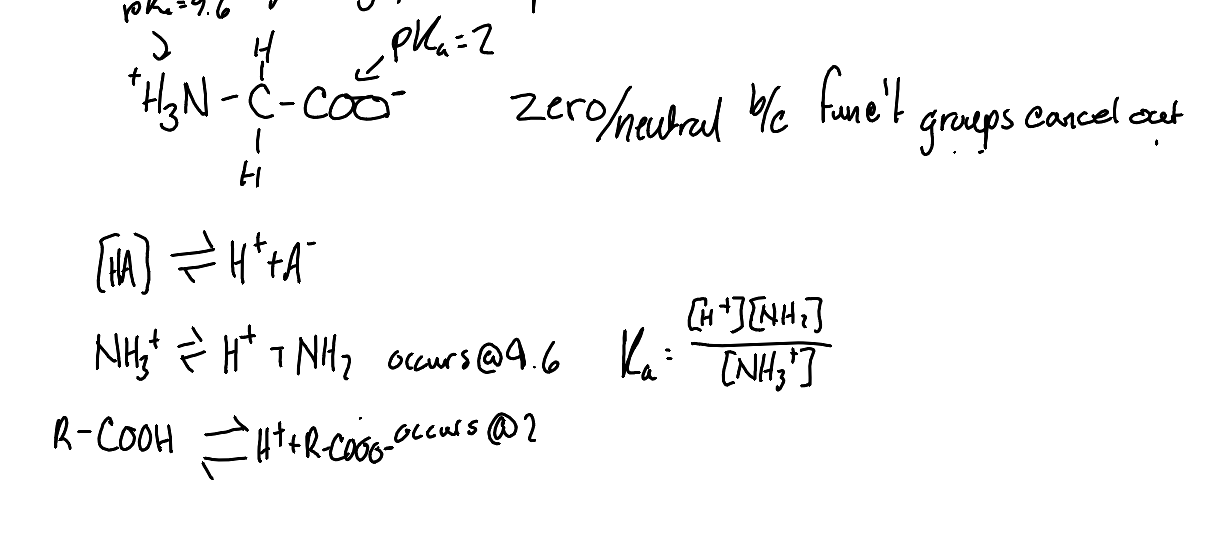
What is the first step in the thought process to solve an amino acid problem?
Identify the ionizable functional groups
What is the second step in the thought process to solve an amino acid problem?
Write out the dissociation reactions assiciated with each pKaand determine their contributions to the overall charge.
What is the third step in the thought process to solve an amino acid problem?
Determine the charge of the functional groups at the given pH values.
What is the fourth step in the thought process to solve an amino acid problem?
Add up all charges over the molecules
What is the cationic form of an enzyme?
The form of the enzyme that carries a net positive charge due to the protonation of its functional groups.
What is the anionic form of an enzyme?
The form of the enzyme that carries a net negative charge due to the deprotonation of its functional groups.
What is a zwitterion (neutral)
An ion that has both positive and negative charges but is overall electrically neutral, typically found in amino acids at physiological pH.
How are amino acids conventionally sorted?
By polarity and charge
Do you think the charge of this molecule across pH range coul change depending on the identity of the R group?
Yes, as long as the R-group has its own ability to accept/donate protons and an entirely different pKa value. This can influence the overall charge of the molecule in different pH environments.
What is the pKa of cysteine?
~8
What is the pKa of selenocysteine?
~5.5
What is the pKa of tyrosine?
~10