chemistry
1/36
Earn XP
Description and Tags
mod 1/2 y11
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
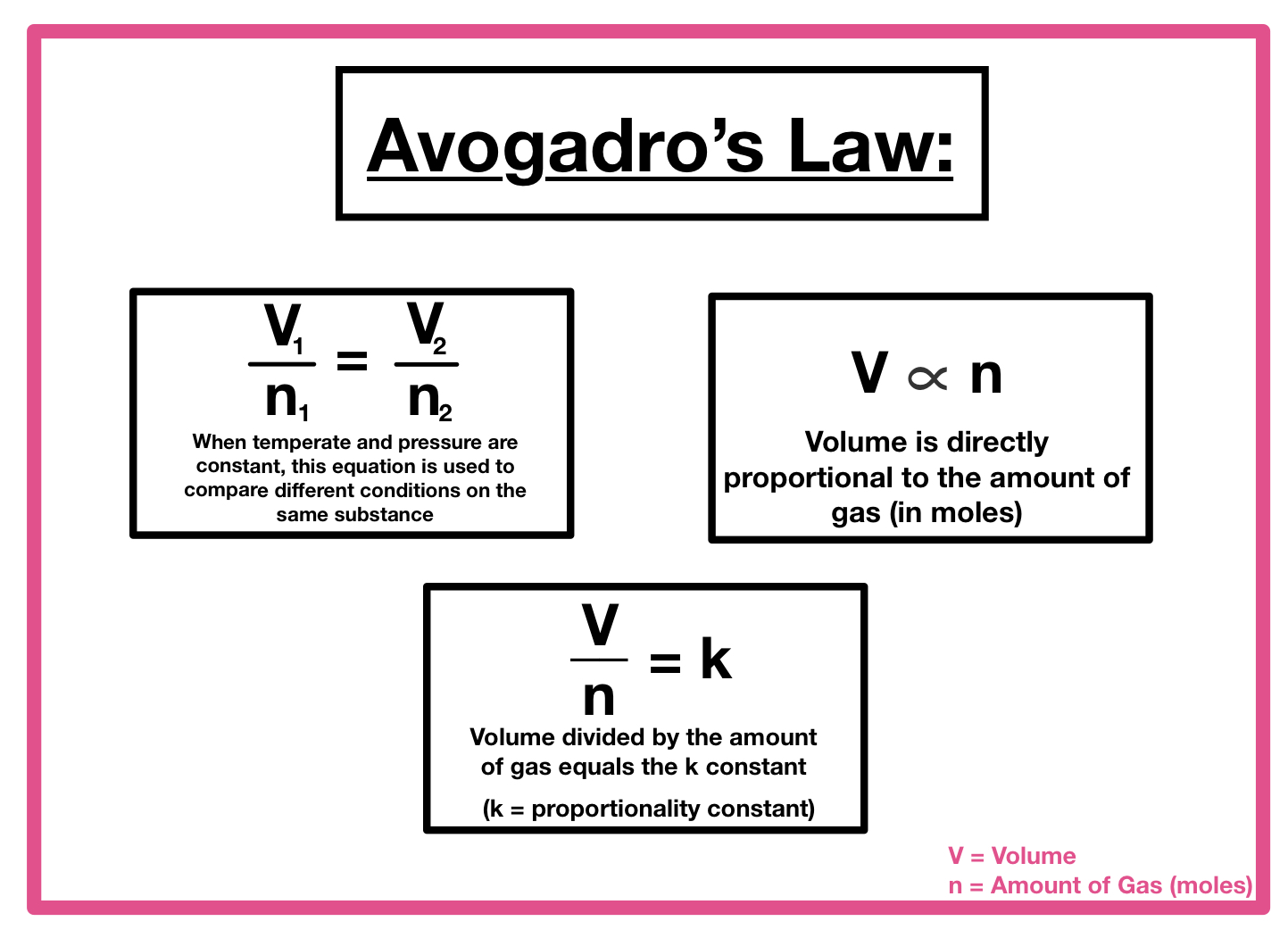
Boyle’s Law
Pressure is inversely related to volume
P1V1 = P2V2
Vice President is a Boy
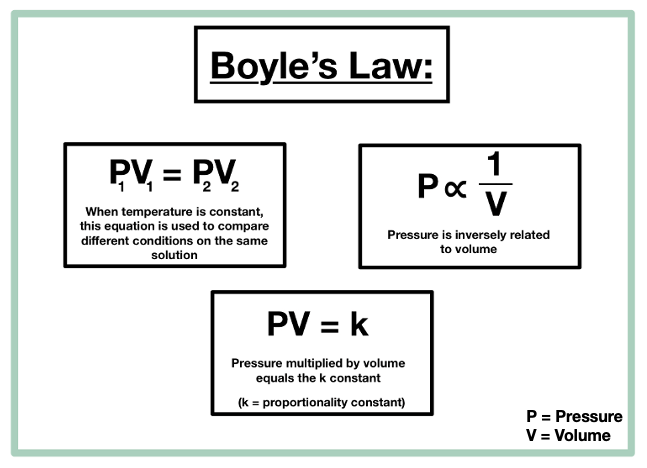
Charles’s Law
Temperature is directly proportional to volume
V1/T1 = V2/T2
Charlie and the chocolate factory on TV
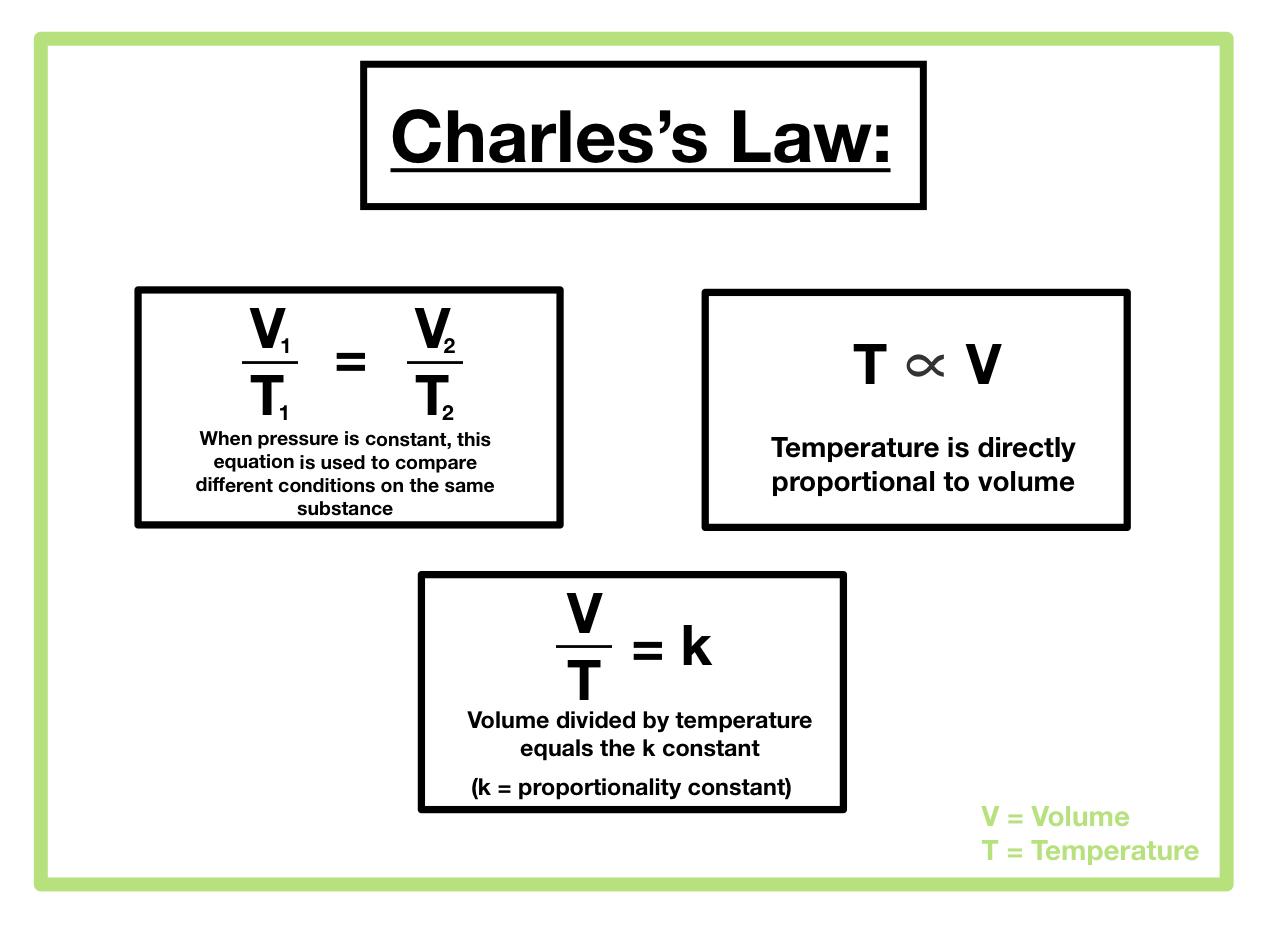
Gay Lussac’s Law
Pressure is directly proportional to temperature
P1/T1 = P2/T2
Personal Trainer is Gay
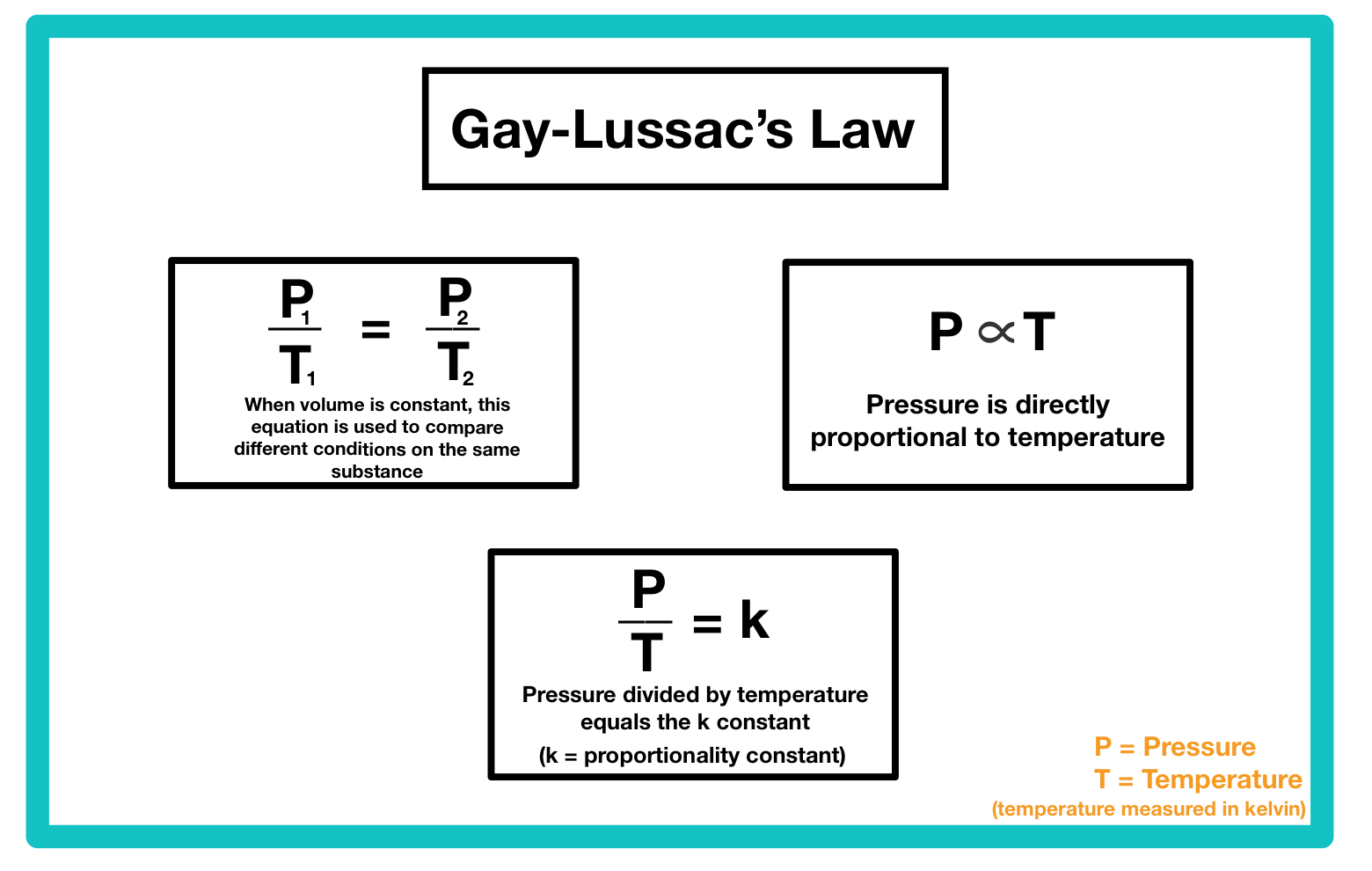
Avogadro’s Law
Volume is directly proportional to the amount of gas (moles)
Equal volumes of all gases, at the same temperature and pressure, have the same number of molecules
Thompson’s Plum Pudding Model (1904)
Negatively charged electrons are embedded within the uniform, positively charged atom
Rutherford’s Gold Foil Experiment
Bombardment of gold foil with alpha particles showed that only some particles were deflected, most passed straight through
Rutherford’s Nuclear Model (1911)
Dense, small, positively charged nucleus with electrons orbiting like planets in individual orbits
Mostly empty space
Bohr Model (1913)
Electrons orbit the nucleus in fixed energy levels
Various energy levels can hold different numbers of electrons
Ground state and excited state electrons
Only explains emission spectra for hydrogen atom
Schrödinger Quantum Mechanical Model (1926)
Assumes the electron is a wave and describes the regions in space, called orbitals, where electrons are most likely to be found
Concept of sub-energy levels
Law of Conservation
Energy is not created nor destroyed, only transferred from one form to another. The total energy of an isolated system remains constant.
Law of Disorder
The entropy of a closed system is always increasing. A reaction will be spontaneous if the products are of higher entropy than the reactants because particles have a tendency to move towards a state of higher disorder and lower energy.
Collision theory
The rate of a chemical reaction increases as the frequency of successful collisions between reactant particles increases. For a successful collision to occur, particles collide with sufficient energy to overcome activation energy and in proper orientation.
Indigenous practices - Cycads
Cycad fruits contain the toxic substance cycasin
Causes vomiting, nausea, and abdominal pains, as well as long-term damage to the nervous system and liver
Very low solubility in water
Cooked → Grated/powdered (increase SA) → Leached
Alpha radiation
Good ionising radiation
Stopped by paper (low penetrating power)

Beta radiation
Fair ionising radiation
Stopped by aluminium foil
Occurs with surplus neutrons

Gamma radiation
Electromagnetic radiation: no particle - high energy & high frequency
Low ionising radiation
Stopped by lead (high penetrating power)
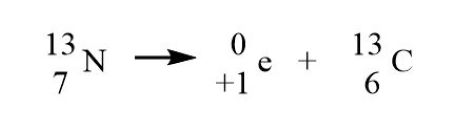
Positron emission (β+)
Occurs with surplus protons
Limewater test
Limewater will turn cloudy in the presence of carbon dioxide gas
CO2(g) + Ca(OH)2(aq) → CaCO3(s) + H2O(l)
Pop test
In the presence of hydrogen gas, a burning splint will be put out with a ‘pop’ sound
H2(g) + 2O2(g) → H2O(l)
Glowing splint test
In the presence of oxygen gas, a smouldering splint will reignite
Oxygen is a key component in combustion → rate of combustion increases in an abundance of oxygen
Solubility rules

Activity series
Isotopes in medicine - Iodine-123
Half-life of 13.22 hours
Used in medical imaging to detect thyroid function and detect adrenal dysfunction
Produced from a cyclotron
Isotopes in Industry - Cobalt-60
High intensity gamma ray emitter - can take industrial x-rays of welding seams to detect flaws in structures
Isotopes in Agriculture - Phosphorus-32
Used in fertilisers to track a plant’s intake of fertiliser from the roots to the leaves, as phosphorus emits beta radiation that can be mapped
Hund’s law
Electrons are placed in individual orbitals in a subshell before doubling up in the same orbital
Pauli’s Exclusion Principle
No more than 2 electrons can occupy the same orbital, two electrons in the same orbital must have opposite spin
Aufbau Principle
Electrons fill orbitals starting at the lowest available energy state before filling in higher states
Properties of an ideal gas
Compressible
Takes shape of container
Always in motion
Collides elastically with container - no energy loss
Intermolecular forces between particles assumed to be zero
Physical properties of Ionic Compounds
Hard - strong, rigid ionic bonds
High m.p, b.p
Brittle - applying pressure shifts alignment of ions that are in a lattice structure
Poor conductors of electricity and heat (solid) - lack of mobile charge carriers as ions are in rigid lattice structure
Good conductors of electricity and heat (aqueous) - ions are free to move
Soluble - in polar solvents (e.g. water), where ion-dipole attraction results in ions of solute being surrounded by molecules of solvent
Physical Properties of Metals
Hard and dense - strength determined by number of valence electrons
Malleable, ductile - metal ions are free to move over each other while maintaining their metallic bonds
Good conductors of electricity and heat - delocalised electrons as mobile charge carriers
Lustrous - delocalised electrons reflect light well
Physical Properties of Covalent Network Substances
Insulators - atoms are neutral and held by strong covalent bonds, electrons are not free to move
Hard and strong - covalent bonds are strong
High m.p and b.p - all covalent bonds need to be broken
Insoluble - polar solvents like water are not attracted to the neutral atoms of a covalent network substance
Physical Properties of Covalent Molecular Substances
Soft and flexible - weak intermolecular forces
Relatively low m.p and b.p - weak intermolecular forces
Many are insoluble, however polar covalent molecular substances (e.g. water) dissolve well in a polar solvent
Do not conduct electricity (aqueous) - dissolve into molecules rather than ions
Indicators of a chemical reaction
Permanent colour change
Formation of a precipitate
Formation of a gas (effervescence)
Production of an odour
Change in temperature
Burning
Production of light
Haber process
Heterogenous catalyst, reversible reaction
Atmospheric nitrogen to ammonia, catalyst of finely divided iron which breaks triple bond of nitrogen
2N2(g) + 3H2(g) 2NH3(g)
Elephant toothpaste
Homogenous catalyst
Possible catalysts:
Saturated KI(aq)
Yeast
KMnO4
MnO2
2H2O2(aq) 2H2O(l) + O2(g)
Contact process
Multi-step process of producing sulfuric acid
Make sulfur dioxide
S(s)+O2(g) SO2(g)
Convert sulfur dioxide → sulfur trioxide via heterogenous vanadium pentoxide catalyst
2SO2(g)+O2(g) 2SO3(g)
Convert sulfur trioxide → concentrated sulfuric acid
H2SO4(l)+SO3(g)→H2S2O7(l) - (oleum)
H2S2O7(l)+H2O(l)→2H2SO4(l)