Multiple choice
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
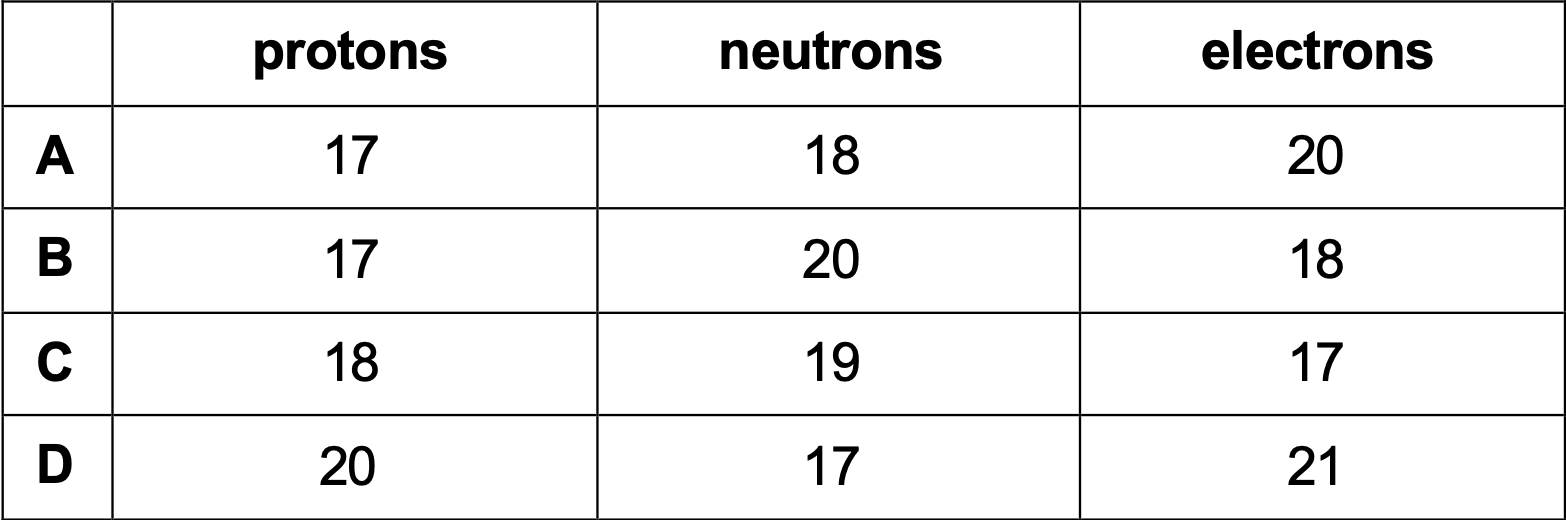
Which row shows the structure of 37Cl- ?
B

What determines the order of elements in the periodic table?
C

What is the number of oxygen atoms in 176.0g of CO2?
D

0.48g of element X, reacts with 0.0200mol Cl2 to form chlorine with the formula XCl4.
What is element X?
D
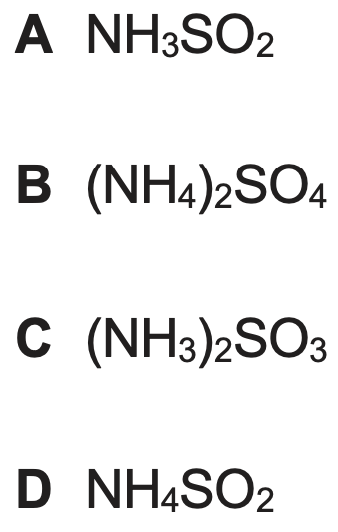
What is the correct formula for ammonium sulphate?
B
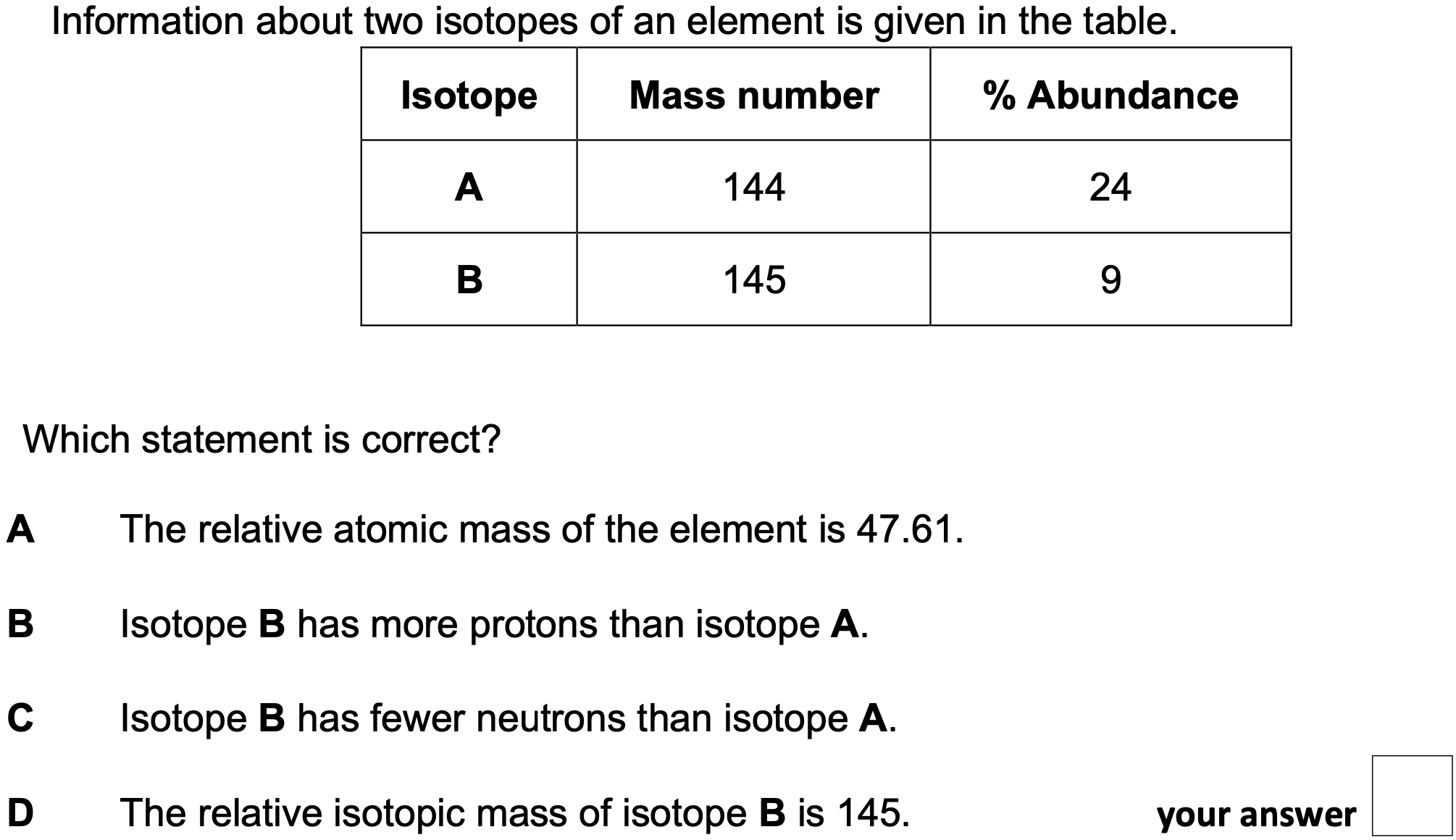
Which statement is correct?
D
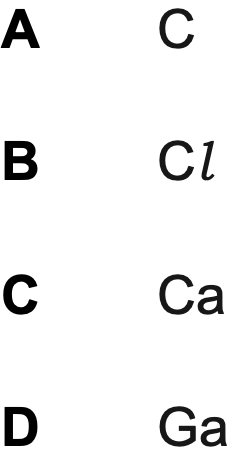
Which element has atoms with the greatest number of singly occupied orbitals?
A
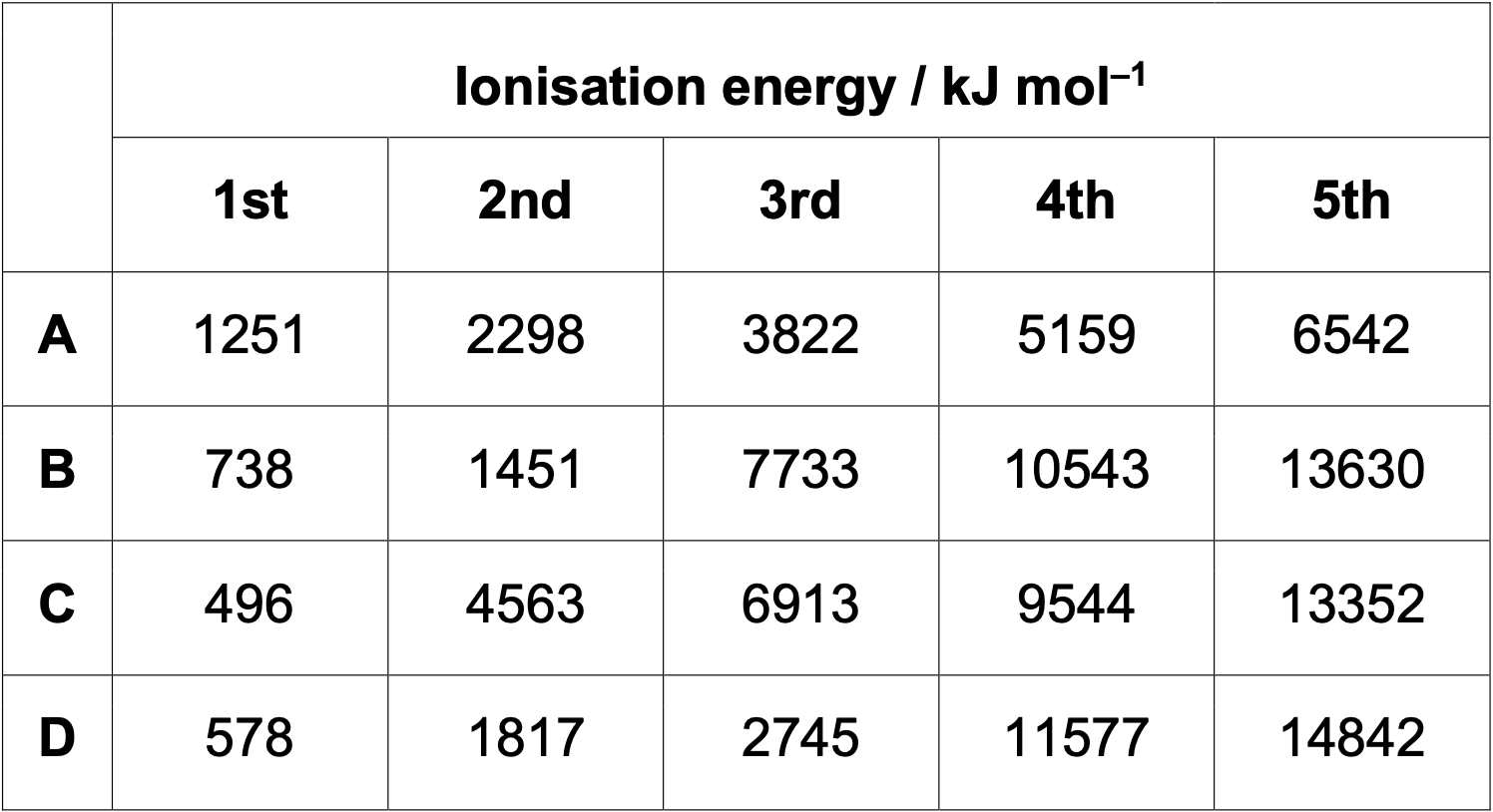
Successive ionisation energies of 4 elements in period 3 are shown below?
B
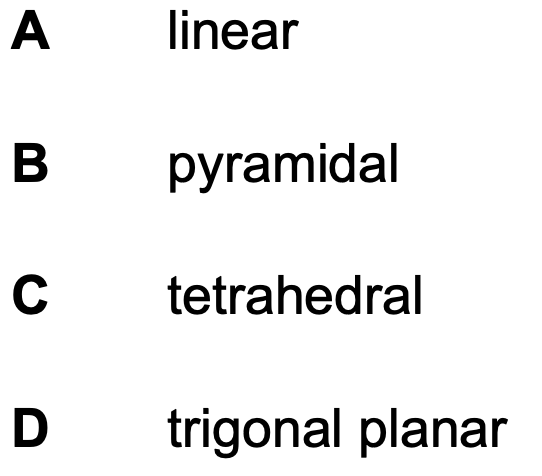
What is the shape around the carbon atoms in graphene?
D
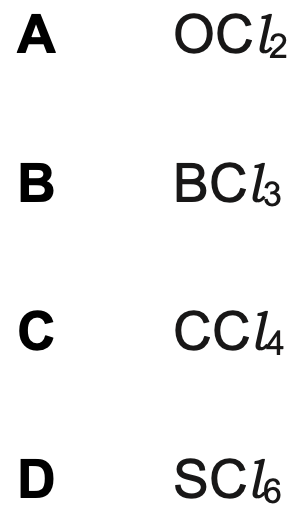
Which compound has polar molecules?
A
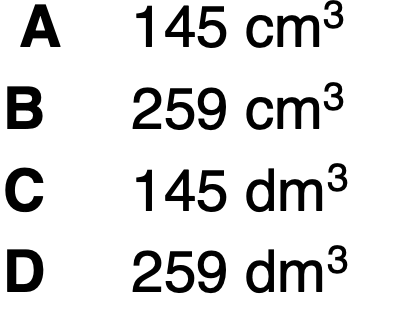
What is the volume of 0.0100mol of N2 at 350oC and 200kPa?
B
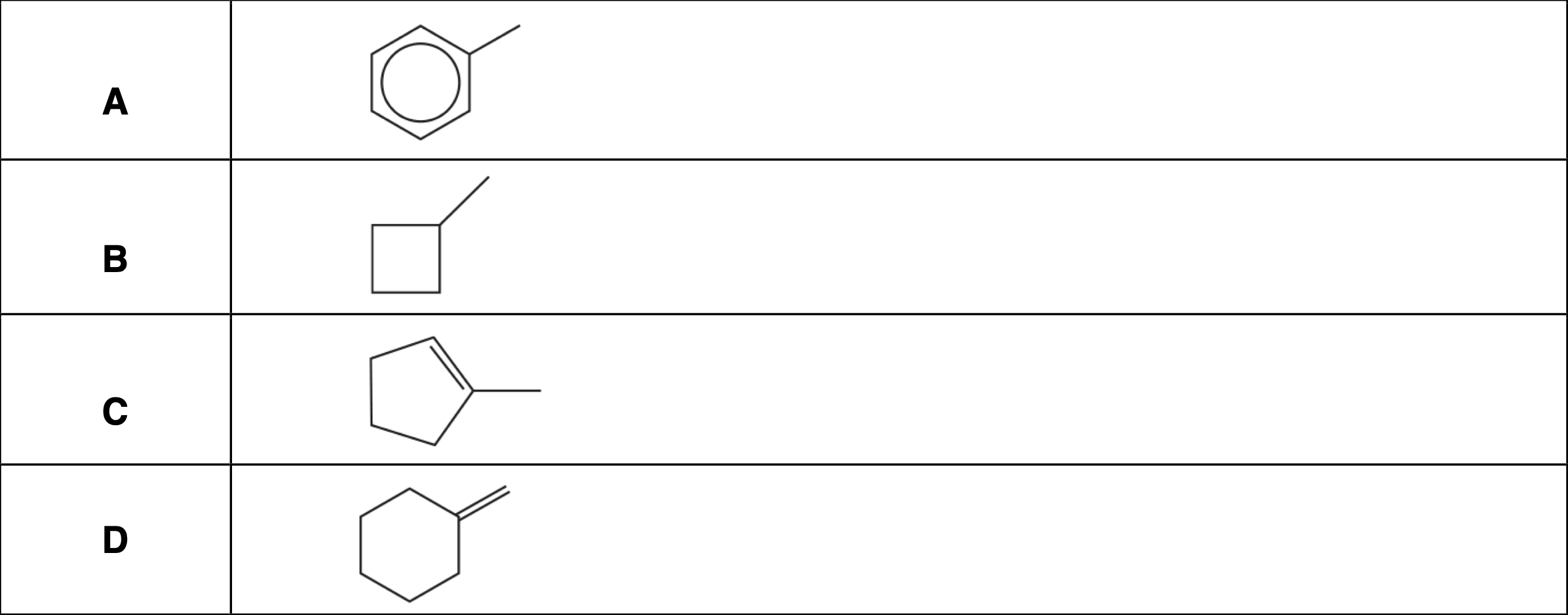
Which compound is unsaturated, alicyclic and contains an alkyl group?
C
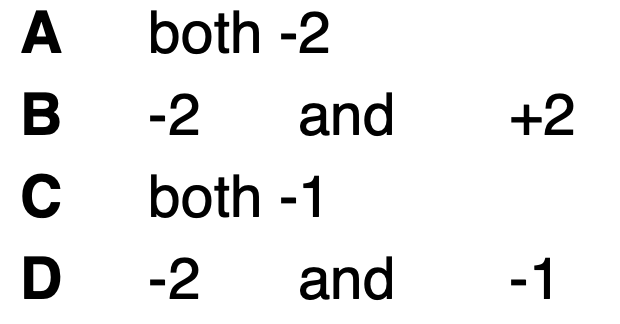
Which are the correct oxidation numbers for the O atoms in these compounds?
CaO and H2O2
D
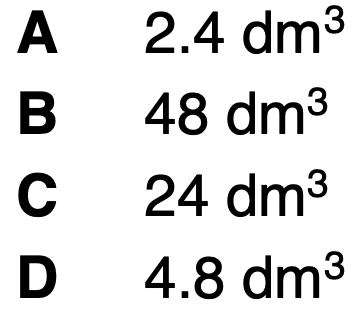
What volume of gaseous product at RTP is formed when 4.00g of hydrogen gas, also at RTP, is burnt in pure oxygen?
B

Which statement is correct?
B
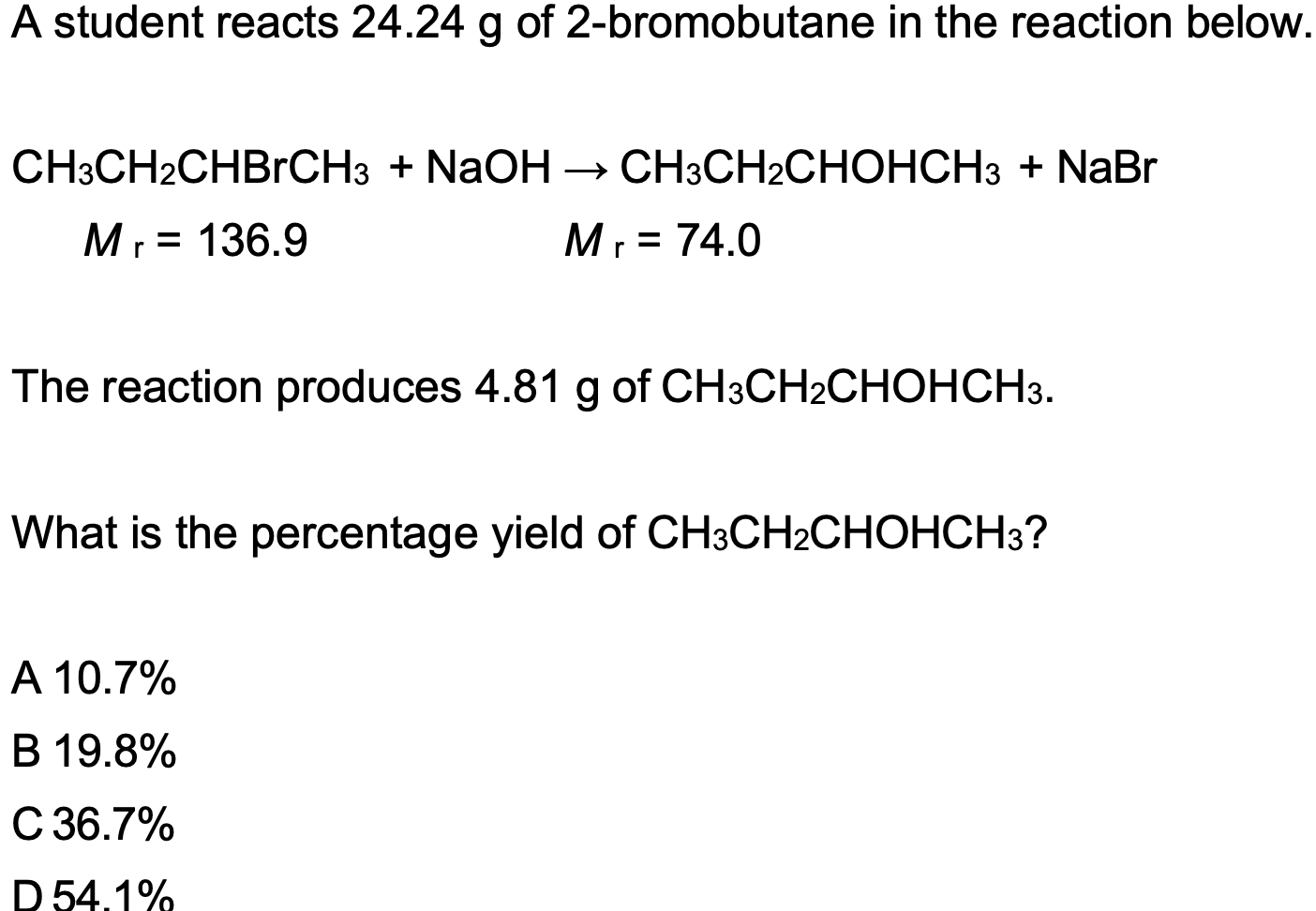
C
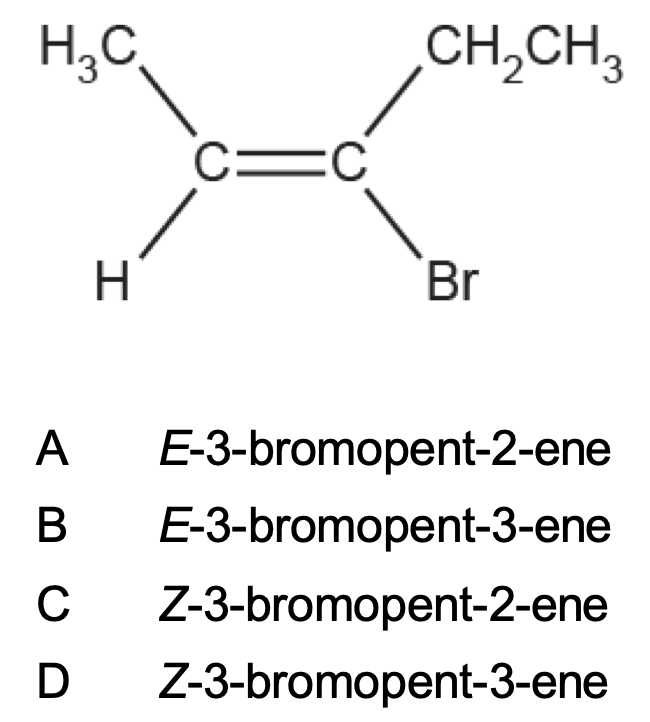
What is the name of the compound below?
A
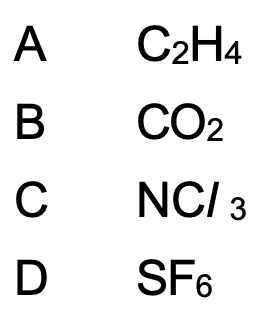
Which substance contains polar molecules?
C

A student carried out an experiment to measure the enthalpy change of combustion of methanol. The energy from the combustion of methanol was used to heat a beaker containing water. The student’s calculated enthalpy change of combustion was more exothermic than the value in the data books.
What error could have made this difference?
B
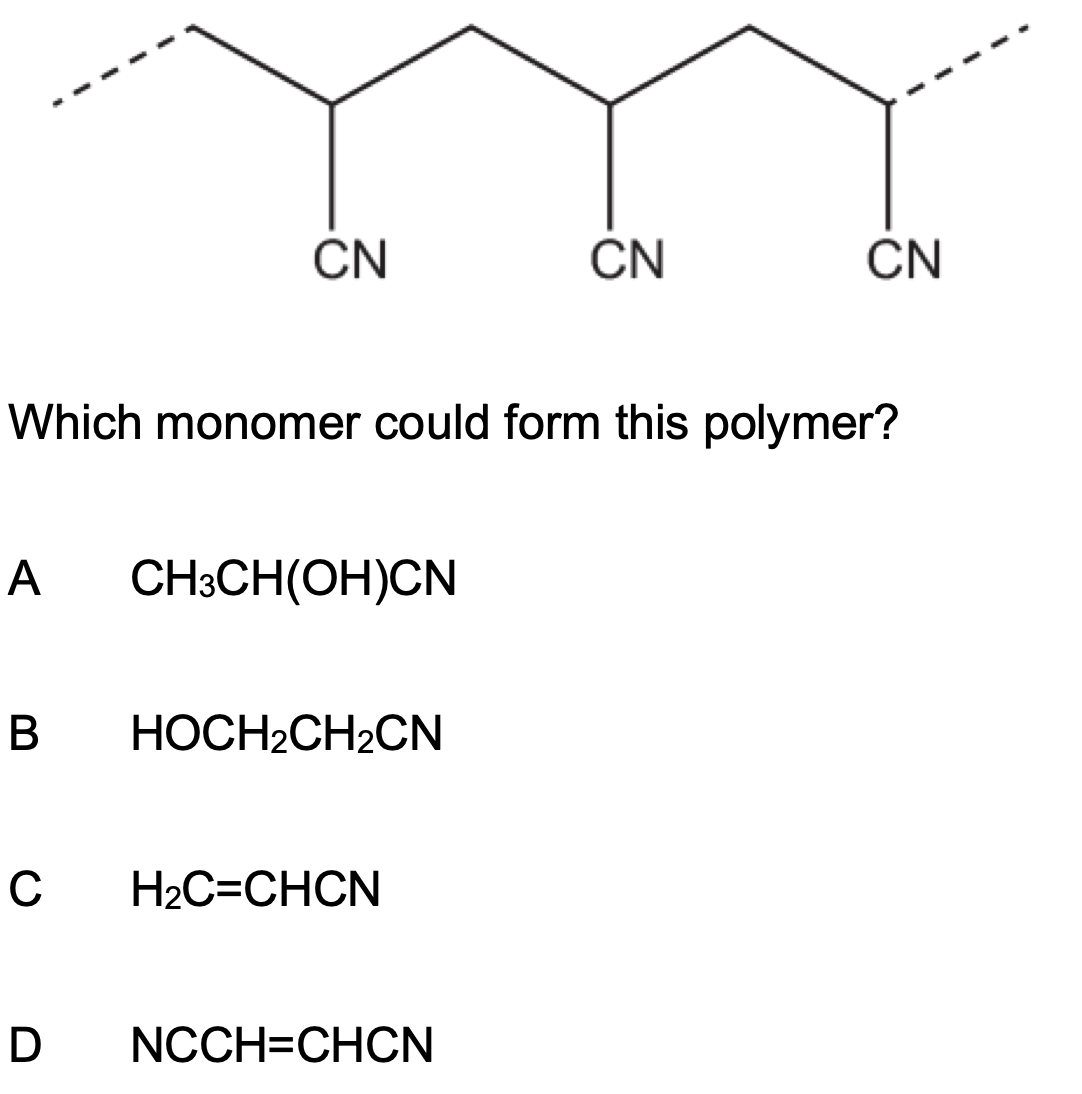
A section of a polymer is shown below.
Which monomer could form this polymer?
C
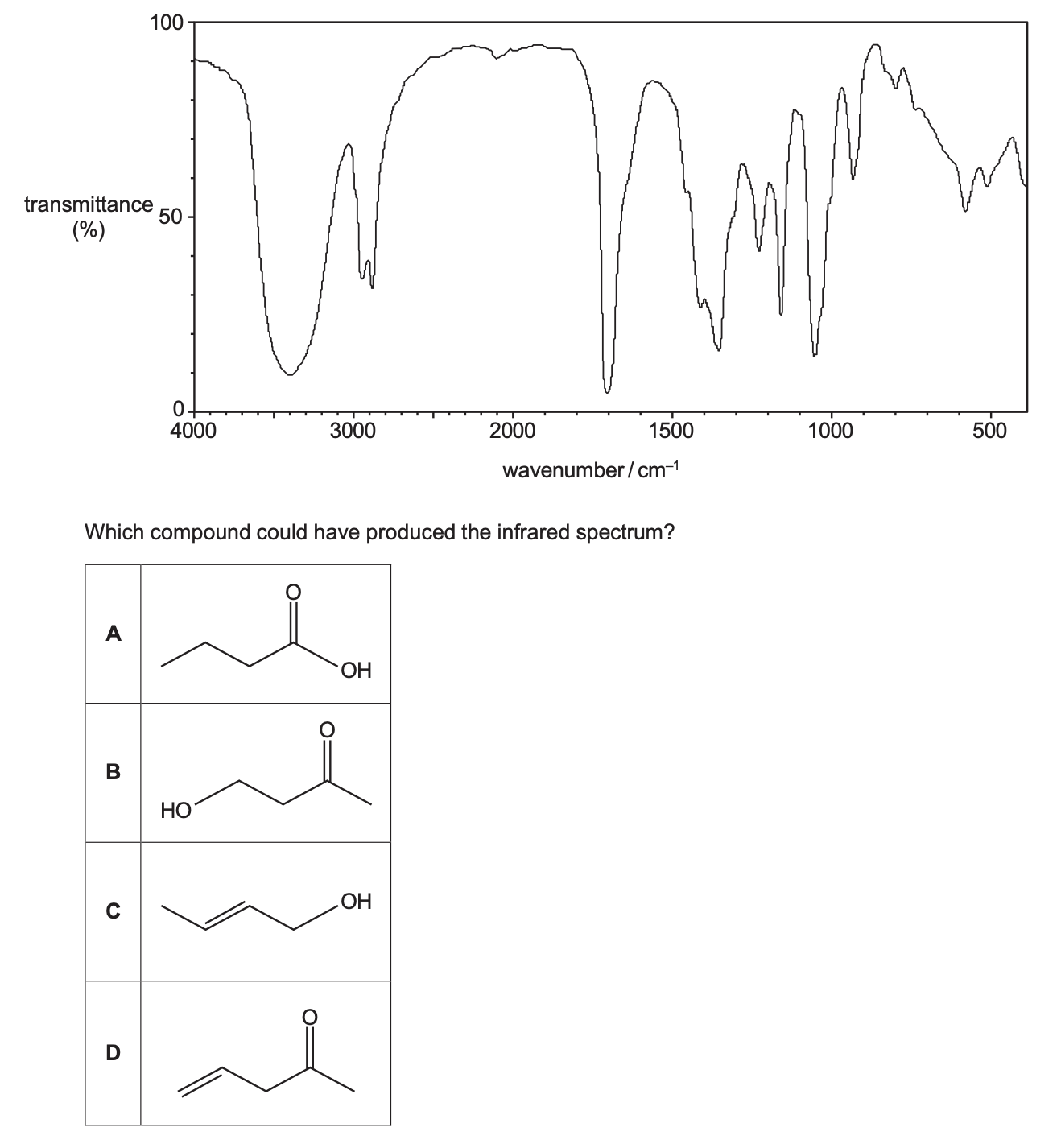
Which compound could have produced the infrared spectrum?
B
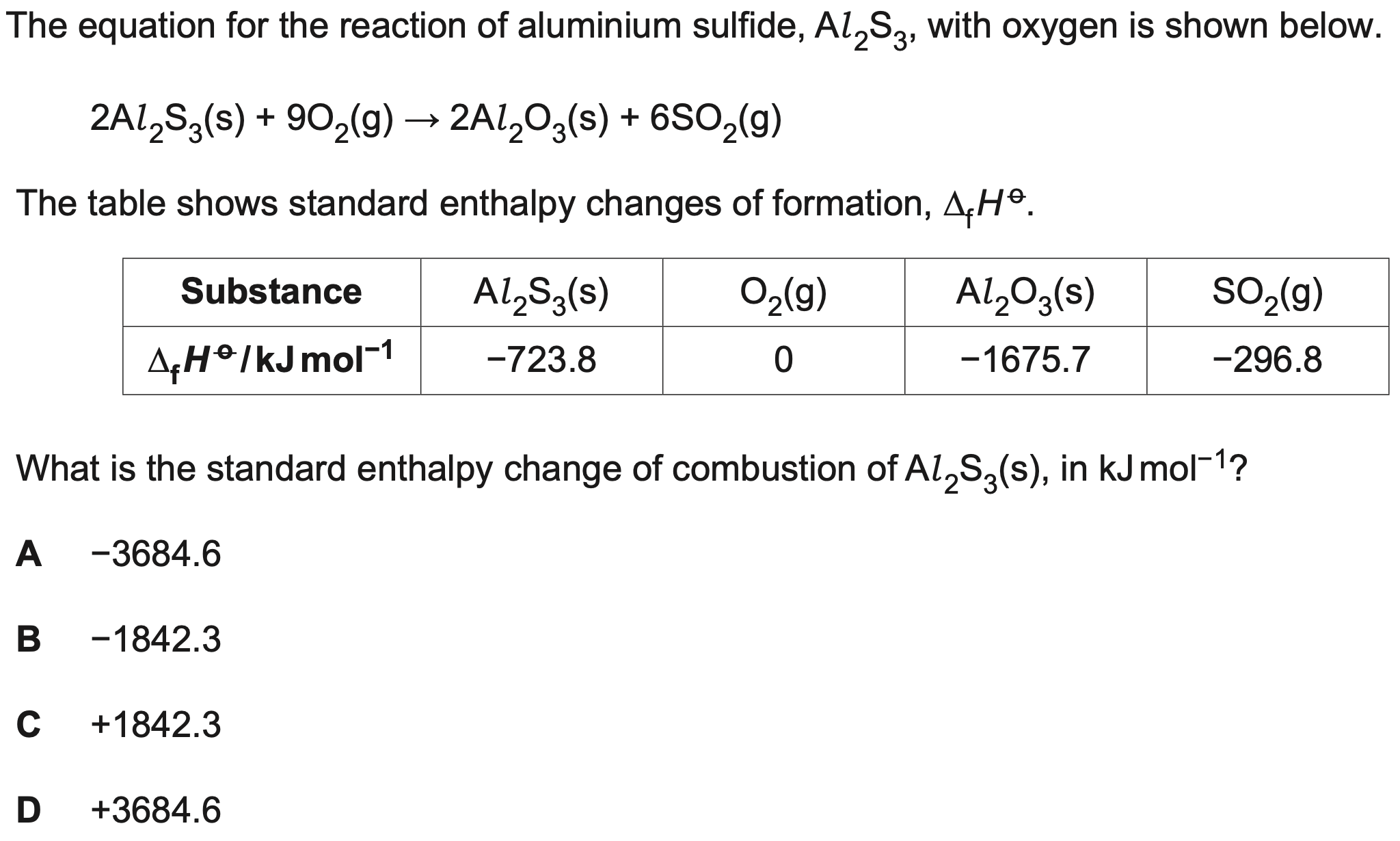
What is the standard enthalpy change of combustion?
B
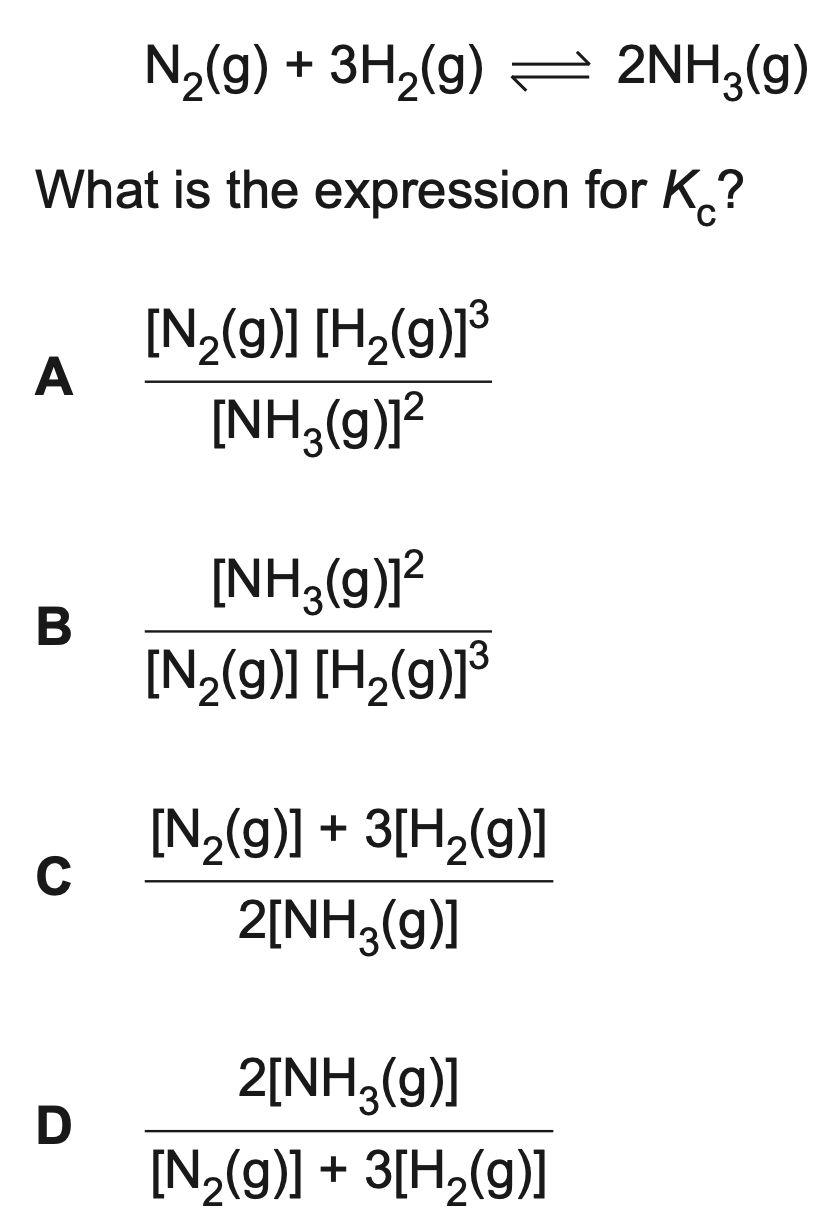
The reversible reaction below is at equilibrium
B
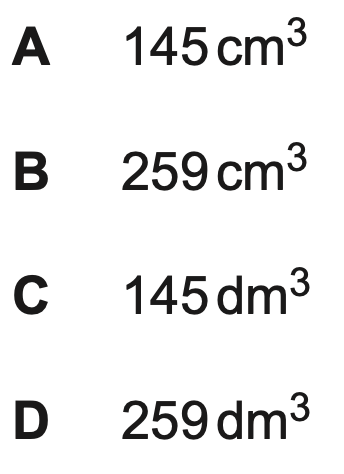
What is the volume of 0.0100mol of N2 at 350oC and 200kPa?
B