Motor Protein and Cilia/Flagella
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
Categories of Motor Proteins
Interaction between microtubules and motor proteins: Kinesins and dyneins
Used in fast axonal transport in neurons or the sliding of MTs in cilia and flagella, transporting membrane organelles mitosis
Interactions between actin microfilaments and members of the myosin motor protein
Muscle contraction, organelle transport, cytokinesis, cell motility, maintaining cell shape
Overview of motor proteins
Converts chemical energy (ATP) in to mechanical energy (force/movement)
It moves unidirectionally in a stepwise manner, It travels to the plus end of microtubules (anterograde direction)
No motor proteins uses intermediate filaments as tracks
Kinesin structure and directional movements
A tetramer, 2 heavy chains and 2 light chains
Has a globular head regions that attaches to microtubules, acts as a ATP-hydrolyzing engine
Uses head to walk on MT
KRP heads are evolutionarily conserved
A neck regions that connects head to the stalk
A coiled helical stalk region that provides flexibility during movement
A light-chain regions that attaches the kinesis to proteins, organelles or other cargo
KRP Tails are highly divergent that reflects the cargo diversity
Kinesins are plus end directed motors
Kinesin Family
Made of 14 kinesin-related protein with different structures and functions
Classified based on their structure
Some are homodimers; heterodimers or tetramers
Majority of Kinesins have the motor domain on the N-terminus
Kinesin 1 is the classic protein most studied
Kinesin 13 have its motor domain in the middle of the protein
Kinesin 14 has a motor domain on the C-terminus causing it to be a minus end directed motor, travels in the retrograde direction
Kinesins are involved in many different cellular processes
Kinesin 1
Dimer, moves cargo to plus end of MT
Kinesin 3
Monomer movement of synaptic vesicles in neurons
Kinesin 5
Bipolar, tetrameric, bidirectional sliding of MTs during anaphase of mitosis
Kinesin 6
completion of cytokinesis
Kinesin 13
Dimer; destabilization of plus ends of MTs
Catastrophins
Kinesin 14
Spindle dynamics in meiosis and mitosis
Moves towards minus ends of MTs
About Movement of Kinesin 1
Kinesin motor step is 8nm from 1 B-tubulin to the next B-tubulin
Globular binding domains, head, from the heavy chains bind to the microtubules
Uses a hand-over-hand model of movement with 2 globular head domains taking turns as the lead hand
1 head remained on the microtubule while 1 head swings over to take a step
It is coupled with ATP hydrolysis
Each kinesin molecule exhibits high processivity
It can move long distances along he MT before detaching
Steps of Kinesin movement
Leading heavy chain binds to ATP
ATP binding causes a conformational change allowing the trialing heavy chain to swing forward
Trailing heavy chains finds a new MT binding site
New leading chain releases ADP and the new trailing head hydrolyzes ATP to ADP and Phosphate
Kinesin-1 role in the cell
Responsible for organelle transport and maintaining the correct organelles’ localization occurs in most cells
Comparing the MTs within the cell and the location of the organelles close to the MT tracks, you can discern the function of the kinesin for that cell
Types of Dynein
Cytoplasmic and Axonemal
Axonemal is in cilia and flagella
Cytoplasmic Dyneins
High processivity toward the minus head of microtubules
Able to catalyze consecutive reaction son a single substrate molecules without releasing it
Structure
2 identical heavy chains that has a force generating head
Protruding stalk
A Binding site for MTs and Tail
Number of light and intermediate chains
ATP hydrolysis causes conformational changes in the motor domain in the linker region that connects the motor domain to the MT-binding domain
Dynein requires an adaptor molecule to interact with the cargo (dynactin and spectrin)
Moves towards the minus end of MT
Roles of Cytoplasmic Dynein
Position the centrosome and Golgi complex and moving organelles, vesicles and particles through the cytoplasm
Position the spindle and move chromosomes during mitosis
Kinesin vs Dyneins
Both move similar materials but in opposite directions on the same railway network (both move on MT)
organelles may bind to both kinesin and dynein simultaneously engaging in a tug a way battle for the cargo
Structure of Cilia
Cilia is 2-10 micrometers long, Most cells have one to many cilia
Occurs in both unicellular and multicellular eukaryotes
Generates a force perpendicular to the cilium like an oar-like pattern
Motile cilia move fluid through tracts by mucus propellers
Non-motile ciliar have sensory function
Flagella
Moves cell through a fluid environment
Is the same diameter as cilia but is much longer (up to 200 micrometers)
Limited to one or few per cell and move with a propagated bending motion
(like a wave)
Force generated is parallel to the flagellum
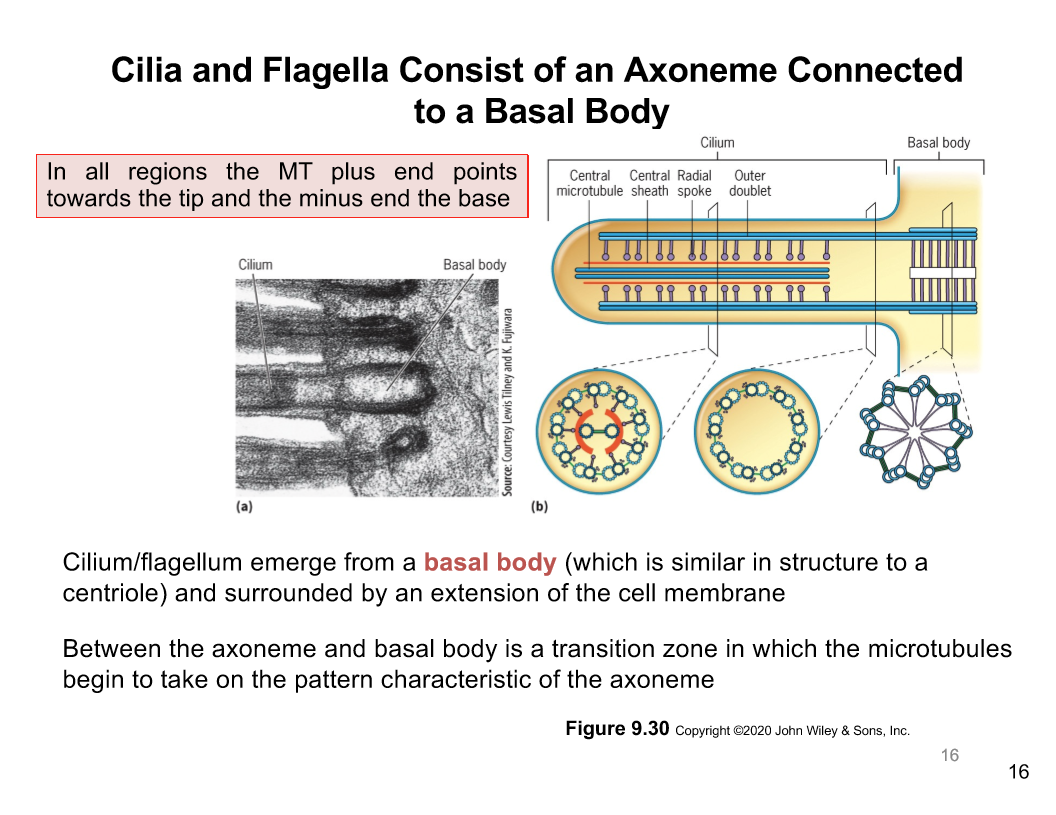
Axoneme in Cilia/Flagella
Cilia and flagella is made of an axoneme connected to the basal body
In all regions the microtubular plus end points towards the tip and the minus ends point to the base
Cilium/flagellum emerger from a basal body like a centrosome
Its surrounded by an extension of the cell membrane
There is a transition zone between axoneme and basal body
Its where the microtubule takes on the characteristic pattern of axoneme
Basal Bodies
It is like the MTOC
consists of 9 triplets MTs
act as a nucleation site for MTs
Cross section of axoneme
Axonemes have a "9+2" pattern with 9 outer doublets (A and B tubules)
1 doublet is complete with 13 subunits
1 incomplete microtubule, 10 or 11 subunits
2 MTs in the centre, central pair
Doublet are connected to one another by a bridge composed of an elastic nexin link
Axonemal dyneins project from the complete MT as pair of arms
Intraflagellar Transport
Assembly and disassembly of the cilium and flagellum require the transport of material to and from the distal
Movement of structural components is intraflagellar transport which occurs between the peripheral doublets and the cell membrane
IFT proteins assemble as linear trains to carry cargo
Kinesin-2 a plus-end-directed motor pulls the IFT trains towards the cilium/flagellum
Cytoplasmic dynein (minus-end directed motor) returns IFT trains to the cell body
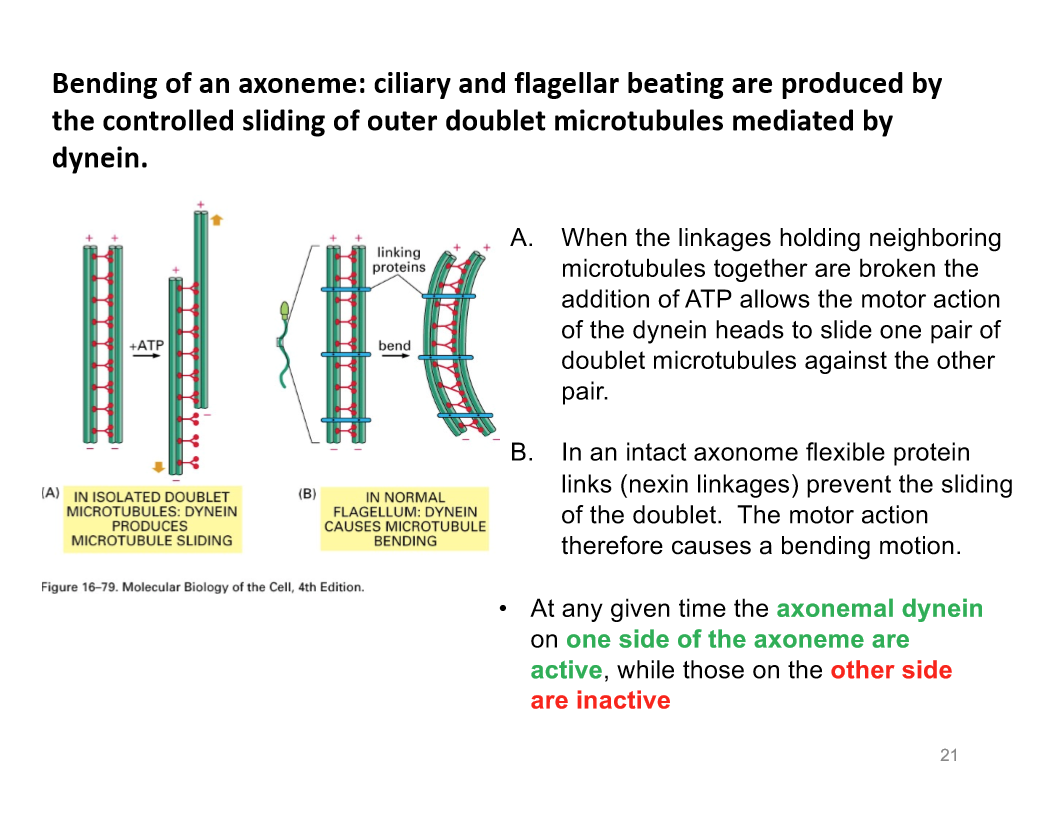
Bending of Axoneme
Axonemal dynein is involved in the sliding of MTs against each other
The stem of each axonemal dynein molecule is anchored to outer surface of A tubule (complete one)
Globular heads/stalk point towards B tubule of the neighboring doublet
The minus-end directed motor axonemal dynein exert force of the neighboring microtubule (B), it pulls the A tubule to the minus end
Like if you are on a boat in the pool (boat is Tubule A) you hold onto the wall (tubule B) to pull yourself along the wall (towards the minus end)
dynein produces microtubule sliding
Linkages holding neighbouring microtubules together are broken. The addition of ATP allows the motor action of dynein heads to slide one pair of doublet MT against the other pair
Dynein causes MT bending
An intact axoneme flexible protein linkage prevent the sliding of the doublet
The actual motion action is a bending motion
At any given time the axonemal dynein on one side of teh axoneme are active while the other side is inactive
Primary Cilia an Non-motile cilia
Primary cilia is used in sensory structures
Important in development; defects can cause disorders like deafness and left-right asymmetry reversals
Non-motile cilia does not have the central pair of MT that the dynein links to is it are unable to move
Motile Cilia Functions in the body
Respiratory system and fertility in reproductive system
Non-motile Function in the body
Eyes
Smell (nose)
Hearing (ears)
Skeletal system
Reproductive
Brain
Kidney
liver