Clin. Pharm. : Adverse Drug Reactions
1/79
Earn XP
Description and Tags
Midterms
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
"A response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function" - WHO, 1972.
Adverse Drug Reactions
An injury resulting from medical intervention that is related to a drug. This can include medication errors, advr and allergies.
Adverse Drug Event
"An appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regime, or withdrawal of the product" - Edwards and Aronson, 2000.
Adverse Drug Reactions
Risks associated with medicinal
documented throughout history:
Digitalis AEs
Thalidomide disaster
Practolol
Rofecoxib
Reason for narrow drug monitoring, for heart
Digitalis AEs
Associated with birth defects (1950s, as sedative and antiemtic for pregnant women but causes phocomelia)
Withdrawn in December 1961
Left 8000-12000 deformed children substances
Thalidomide disaster
____- cardioselective ß-blocker 4 years in the market
Initially associated with rashes, some severe
Psoriasis-like rashes linked to dry eyes, including irreversible scarring of the cornea
Sclerosing peritonitis, a bowel condition associated with significant mortality
Practolol
Practolol effects
Psoriasis-like rashes
Sclerosing peritonitis
Increased risk of cardiovascular events; thrombotic risk.
Withdrawn in 2004
Triggered post marketing surveilance
Rofecoxib
ASSESSING DRUG SAFETY
> When drugs are newly introduced to the market, their
safety profile will be ___(not fully established).
> Pharmacists as well as healthcare professionals take an
active role in monitoring, reporting, and trending ADR
information
PROVISIONAL
CLASSIFICATION OF ADR
Rawlin-Thompson
DoTS system
Rawlin-Thompson divides into what 2 main groups?
Type A and Type B
What type of Rawlin-Thompson classification?
Normal but quantitatively exaggerated pharmacological effects of a drug.
Most common
Predictable and Dose-dependent (increases with higher doses)
Ex. Hyperglycemic drugs, antidiabetic drugs; Bleeding from warfarin; Hypotension to anti-hypertensive agents;Severe sedation from antihistamines
Type A
What type of Rawlin-Thompson classification?
Qualitatively abnormal effects which appear unrelated to the drug's normal pharmacology.
More serious in nature, more likely to cause deaths, and are often not discovered until after a drug has been marketed.
Unpredictable and not dose-dependent
- Ex. Hepatotoxicity from isoniazid
Type B
What type of Rawlin-Thompson subtype in Type A (augmented)?
related to pharmacological activity of the drug
Examples:
hypoglycemia (oral sulfonylureas);
tachycardia (albuterol);
diarrhea (antibiotics);
sedation (CNS depressants);
bleeding (anticoagulants)
EXTENSION EFFECTS
What type of Rawlin-Thompson subtype in Type A (augmented)?
unrelated to pharmacological activity of the drugs
predictable,dose-dependent, mild, reversible, may disappear with continued use, or require dose adjustment, part of drugs known safety profile
Examples:
constipation(opiates);
headache (nitroglycerin)( due to vasodilation effect);
dry cough (ACE inhibitor)(due to accumulation of bradykinin);
antihistamine (sedation)
SIDE EFFECTS
What type of Rawlin-Thompson subtype in Type B (Bizzare)?
genetically determined reactions
Examples
Malignant hyperthermia (antipsychotic agents);
hemolytic anemia (G6PD + Antimalarials or sulfonamides);
SJS (Carbamazepine, Phenytoin, sulfonamide)
IDIOSYNCRACY
What type of Rawlin-Thompson subtype in Type B (Bizzare)?
Immune responses to environmental antigens resulting in
symptomatic reactions upon secondary exposure to the same
antigen
HYPERSENSITIVITY REACTIONS
What type of Rawlin-Thompson subtype in Type C (Continuous)?
A condition where a person takes a drug compulsively, despite potential harm to themselves or their desire to stop
Cause chronic relapsing disorder-may lead to loss of control, craving
Examples:
Marijuana
Opiates
ADDICTION
What type of Rawlin-Thompson subtype in Type C (Continuous)?
compulsion to take the drug repeatedly and experiences
unpleasant symptoms if discontinued
tremors,anxiety, sweating
drugs used habitually and the body has become accustomed to its effects
The person must continue use to feel normal or else its symptoms will trigger withdrawals
Examples:
Benzodiazepines (withdrawal causes insomnia, anxiety, or seizure)
Caffeine (withdrawal causes headache, irritability, fatigue)
Cocaine (intense psychological craving and mood disturbances upon withdrawal)
DEPENDENCE
characterized by emotional or mental preoccupation with the drug’s effect and craving for continued use. Developed through prolonged chronic use.
Occurs when drug is used habitually and the mind becomes emotionally reliant on its effect either to illicit pleasure or lift pain or does not feel capable or functioning without it.
Psychological dependence
What type of Rawlin-Thompson subtype in Type C (Continuous)?
Reduced effect with repeated use of drug, need for higher doses to produce the same effect.
examples: Benzodiazepines
TOLERANCE
What type of Rawlin-Thompson subtype in Type D (Delayed)?
ability of any substance to cause or induce cancer (after prolonged exposure)
does not occur right away, years
Examples:
antineoplastic agents-secondary cancer
heterocyclic amines-formed during high temperature of cooking
aromatic hydrocarbons- benzene cause cancer
nitrosamine- in preserved or smoked foods
aflatoxin- from aspergillus species, caused from contaminated grains or nuts
CARCINOGENICITY
What type of Rawlin-Thompson subtype in Type D (Delayed)?
ability of any substance to cause congenital malformities or birth defects
There are drugs that should not be taken by pregnant women
Examples:
carbamazepine and Valproic acid (neural tube defects);
diethylstilbestrol (increased risk of developing vaginal adenocarcinoma after puberty);
phenytoin (fetal hydantoin syndrome- growth deficiency and facial malformation.)
streptomycin (8 nerve damage);
tetracycline (discoloration and defects of teeth and altered bone growth);
thalidomide
phocomelia
isotretinoin
TERATOGENICITY
Neural Tube Defects
Spina bifida,Anencephaly,Encephalocele
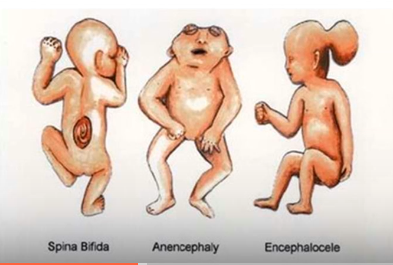
Neural Tube Defects: Incomplete closre of the spinal column leading to nerve damage and paralysis
Spina bifida
Neural Tube Defects: Absence of major portions of the brain and skull, often very fatal
Anencephaly
Neural Tube Defects: Characterized by having sac like protrusion of brain tissue through an opening of brain skull
caused by valproic acid and carbamazepine
Encephalocele
Neural tube defects can be avoided through taking ___
folic acid supplementations
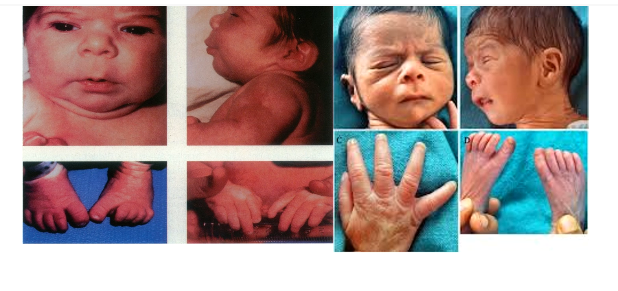
What condition is this?
Low birth weight
Short stature
Microcephaly
Cleft lip
Flat nasal bridge
hypoplastic nails/phalanges- there is absence of distal phalanges
Developmental delay and cognitive deficits
Fetal Hydantoin syndrome
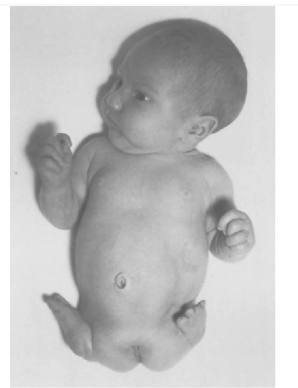
What condition is this?
Rare congenital deformity, the hands and feet are attached close to the trunk. Limbs are closely underdeveloped are absent.
Side effect of thalidomide
Phocomelia
What type of Rawlin-Thompson?
Withdrawal symptoms
Generally occur shortly after stopping a drug
Examples:
opiate withdrawal;
rebound insomnia and excitation (benzodiazepines);
rebound hypertension (Clonidine);
rebound congestion (nasal decongestant)
adrenal crisis (steroids)
Type E (End of Use)
What type of Rawlin-Thompson?
May result from:
Drug-drug interactions
Use of counterfeit drugs
Drug instability
Patient's non-compliance
Wrong route of administration
Drug resistance
Type F (Failure of Efficacy)
The DoTS system is based on ___
Dose relatedness, Timing, and patient Susceptibility
It first considers the dose of the drug, after which the time course of a drug's presence at the site of action, and then the final aspect of classification would be susceptibility.
The DoTS system
The DoTS system:
Relationship of the drug and the occurence of ADRs
Dose relatedness
The DoTS system:
Toxic effects: ADRs that occur at doses higher than the usual therapeutic dose
Collateral effects: ADRs that occur at standard therapeutic doses
Hypersusceptability reactions: ADRs that occur at sub-therapeutic doses in susceptible patients
Dose relatedness
The DoTS system:
occur in relation tho the start or stop of drug theraphy.
Help differentiate immediate reactions, delayed reactions, and withdrawal effects
Time relatedness
The DoTS system:
Time-independent reactions: ADRs that oocur at any time during treatment.
Time-dependent reactions: Rapid reactions occur when a drug is administered too rapicly. Early reactions occur early in treatment then abate with continuing treatment (tolerance). Intermediate reactions ocour after some delay, but if reaction does not occur after a certain time, little or no risk exists. Late reactions risk of ADR increases with continued-to-repeated exposure, including withdrawal reactions. Delayed reactions cocur some time after exposure, even if the drug is withdrawn before the ADR occurs.
Time relatedness
The DoTS system:
refers to individual patient factors that increases the likelihood of an ADR which can be influenced by age, genetics,sex,gender, and diseases states, concurrent medications, lifestyle factors
Susceptibility
The DoTS system:
susceptibility may be present in some individuals, but not others. Alternatively, susceptibility may follow a continuous distribution - increasing suscaptibility with impairod renal function.
Factors include: genetic variation, age, sex, altered physiology, exogenous factors (interactions) and disease.
Susceptibility
Factors affecting susceptibility to ADR
Age
Gender
Co-morbidities and concomitant medicines use
Ethnicity
Pharmacogenetics
Erythrocyte G6PD deficiency
Porphyrias
What susceptibility factor?
Elderly patients - more prone to ADRs, has altered body compositions(renal,hepatic functions)
Changes in physiology
Multiple co-morbidities
Polypharmacy
Children and young adults
Neonatal differences
Underdeveloped physiology
Dosing errors and lack of evidence for both safety and efficacy
Age
What susceptibility factor?
Women may be more susceptible to ADRs.
due to a combination of biological, hormonal, and pharmacokinetic factors. Higher body fat percentage which can alter drug distribution. Anxiety, insomnia, mood changes are more common.
Examples:
Mefloquine - impairment of concentration and
psychiatric adverse events
Cardiovascular drugs (Sotalol) & Erythromycin - Torsades de pointes, a ventricular arrhythmia linked to ventricular fibrillation and death. (women are more susceptible due to naturally longer qt interval and different cardiac depolarization patterns)
Gender
What susceptibility factor?
Reduction in hepatic and renal function substantially increase the risk of ADRs.
Comorbidities: CHF, DM, and peripheral vascular, chronic pulmonary, rheumatological, hepatic, renal, and malignant diseases(cancer) are strong predictors of ADRs.
Co-morbidities and concomitant medicines use
What susceptibility factor?
Due to inherited traits of metabolism
CYP450 has varied distribution among people of differing ethnicity.
CYP450 play major role in drug metabolism.Differs in ethnic groups
Due to genetic polymorphism, would result individuals being clasified as poor metabolizers, leading to slower drug clearance, with increase risk of drug toxicity. Others have extensive or ultra rapid metabolizers leading faster metabolism and reduced therapeutic effects.
Ethnicity
What susceptibility factor?
Examples:
White individuals have poorer warfarin metabolism → Increased risk of toxicity
Black patients using ACEl → Increased risk of angioedema
White & Black patients has greater risk to experience CNS ADRs associated w/ mefloquine than in patients of Chinese or Japanese origin.
Examples: Asian patients using Rosuvastatin has increased risk of myopathy
Susceptibility based on ___could be associated with genetic or cultural factors.
Ethnicity
What susceptibility factor?
It is the study of genetic variations that influence an individual's response to drugs, and examines polymorphisms that code for drug transporters, drug-metabolizing enzymes and drug receptors.
Helps explain why individuals respond differently to the same drug guiding the personalized therapy to improve efficacy and reduce risk of ADRs
Pharmacogenetics
What susceptibility factor?
G6PD deficiency is a sex-linked inherited enzyme deficiency, leading to susceptibility to hemolytic anemia.
Patients with low levels of G6PD are predisposed to hemolysis with oxidant drugs such as Primaquine, Sulfonamides, and Nitrofurantoin.
Erythrocyte G6PD Deficiency
G6PD are predisposed to___
hemolytic anemia
condition in which red blood cells are destroyed and removed from the blood stream before their normal life span is over
even before 120 days, the red blood cells are removed.
hemolytic anemia
normal life span of red blood cells from the blood streams
120 days
What susceptibility factor?
____refers to a group of disorders that result from a buildup of natural chemicals that produce porphyrin in the body.
Porphorin - very essential to the function of hemoglobin. Importnat in the synthesize heme
The effects of drugs are of most importance with acute ___, in which certain commonly prescribed agents may precipitate life-threatening attacks.
These attacks may be presented by severe abdominal pain, vomiting, hypertension, tachycardia, and neurological symptoms
Examples of drugs that cause acute porphyria:
barbiturates
sulfonamides
antibiotics
antiepileptic drugs (phenytoin, carbamazepine, rifampicin, and alcohol)
Porphyrias

What condition is this?
They look like vampires
Porphyria
The immune response is not related to the pharmacological action of the drug and prior exposure to the drug is required.
Allergic reactions range from rashes, serum sickness, and angioedema to life-threatening bronchospasm and hypotension associated with anaphylaxis.
IMMUNOLOGICAL REACTIONS
are immune mediated reactions that are not related to the drug pharmacological action.
Only occur in individuals who have been previously exposed to the drug. Allowing the body to become sensitized.
Allergic reactions or Hypersensitivity reaction
What immunological or hypersensitivity reaction?
Mechanisms: Drug/IgE complex to mast cells release of histamine and leukotrienes.
Symptoms/signs and examples: Pruitis, urticaria, bronchoconstriction, angioedema, hypotension, shock, for example, penicilin anaphylaxis.
Type I (immediate)
What immunological or hypersensitivity reaction?
Anaphylactic type reaction
most common type of allergic type reaction
Triggers when an antigen binds to ImmuneglobulinE antibodies attached to na mast cells or basophils
Type I (immediate)
What immunological or hypersensitivity reaction?
Mechanisms: IgG and complement binding to (usually) red blood cell. Cytotoxic T-cells lyse the cell.
Symptoms/signs and examples: Haemolytic anaemia and thrombocytopaenia, for example, associated with cephalosporins, penicilins and rifampicin. Aplastic anemia caused by chloramphenicol.
Type lI (cytotoxic)
What immunological or hypersensitivity reaction?
Antibody mediated hypersensitvity reaction
mediated by the IgG antibodies
directed against antigens on cell membranes
leads to complement activation leading to cell lysis or phagocytosis
Type lI (cytotoxic)
What immunological or hypersensitivity reaction?
Mechanisms: Drug antigen and IgG or IgM form immune complex, attracting macrophages and complement activation.
Symptoms/signs and examples: Cutaneous vasculitis, serum sickness, for example, associated with chlorpromazine and sulphonamides.
Type III (Immune complex)
What immunological or hypersensitivity reaction?
immune complex mediated hypersensitivity reaction
caused by deposition of antigen body complexes and tissues
activating complements and attracting neutrophils leading to inflammation and tissue injury
IgG or IgM
Type III (Immune complex)
What immunological or hypersensitivity reaction?
Mechanisms: Antigen presentation with major histocompatibility complex protein to T Cells and cytokine and inflammatory mediator
release.
Symptoms/signs and examples: Usual occur after 7-20 days. Macular rashes andorgan failure, including Stevens-Johnson syndrome and toxic epidermal necrolysis, for example, associated with neomycin and sulphonamides.
Type IV (delayed type)
What immunological or hypersensitivity reaction?
cell-mediated hypersensitvity reaction
mediated by Tlymphocytes than antibodies
Occurs in 7 to 20 days
Type IV (delayed type)
PHARMACOVIGILANCE AND EPIDEMIOLOGICAL METHODS IN ADR DETECTION EXAMPLES
Spontaneous reporting
Yellow card scheme
Published case reports
Cohort studies
Case-control studies
PHARMACOVIGILANCE AND EPIDEMIOLOGICAL METHODS IN ADR DETECTION
It collects data about suspected ADRs in a central database.
Cases are not collected in a systematic manner, but accumulate through reports submitted spontaneously by people who make a connection between a drug and a suspected drug-induced event.
Helps in the early detection of new, rare, or serious ADRs
Serves as the foundation of post marketing surveillance
Spontaneous reporting system
PHARMACOVIGILANCE AND EPIDEMIOLOGICAL METHODS IN ADR DETECTION
The assessment of whether a drug is responsible for a suspected ADR is of great importance in both the regulatory environment and within the pharmaceutical industry.
Helps determine the likelihood that a particular drug cause the observed reaction, guiding the regulatory decisions, risk management, and the clinical practice
Causality assessment
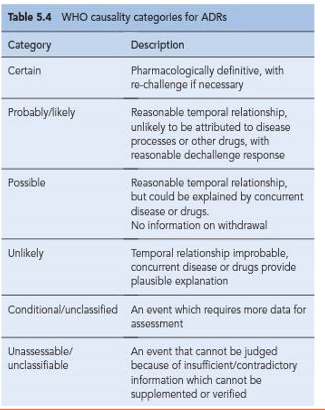
WHO casualty categories for ADRs
Certain
Probably/Likely
Possible
Unlikely
Conditional/unclassified
Unassessible/unclassifiable
What World Health Organization Uppsala Monitoring Centre causality categories?
Pharmacologically definitive, with rechallenge if necessary
Certain
What World Health Organization Uppsala Monitoring Centre causality categories?
Reasonable temporal relationship; unlikely to be attributed to disease processes or other drugs, with reasonable dechallenge response
Probably/Likely
What World Health Organization Uppsala Monitoring Centre causality categories?
Reasonable temporal relationship, but could be explained by concurrent disease or drugs; no information on withdrawal
Possible
What World Health Organization Uppsala Monitoring Centre causality categories?
Temporal relationship improbable; concurrent disease or drugs provide plausible explanation
Unlikely
What World Health Organization Uppsala Monitoring Centre causality categories?
An event which requires more data for assessment
Conditional/ unclassified
What World Health Organization Uppsala Monitoring Centre causality categories?
An event that cannot be judged because of insufficient/contradictory information which cannot be supplemented or verified
Unassessable/ unclassifiable
PHARMACOVIGILANCE AND EPIDEMIOLOGICAL METHODS IN ADR DETECTION
Case reports have been described as a form of non- systematic voluntary reporting.
Reports are not solicited and their appearance in the medical literature is in the gift of medical editors.
depend on the initiatives of health care professionals who observe and document possible adrs
Published case reports
PHARMACOVIGILANCE AND EPIDEMIOLOGICAL METHODS IN ADR DETECTION
___studies are prospective pharmaco- epidemiological studies that monitor a large group of patients taking a particular drug over a period of time.
It can indicate the relative risks associated with the AE in people exposed to the drug being studied.
Cohort studies
PHARMACOVIGILANCE AND EPIDEMIOLOGICAL METHODS IN ADR DETECTION
____ studies are retrospective studies that compare the extent of drug usage in a group of patients who have experienced the adverse event with the extent of usage among a matched control group who are similar in potentially confounding factors, but have not experienced the event.
Case-control studies
Drugs must always be considered as a possible cause of disease or symptoms that are among a patient's list of complaints.
Complete drug histories, including non-prescription drugs, must also be carried out.
Recognition is often subjective, and it is not always possible to demonstrate strong causality between the drug and the occurrence
IDENTIFYING AND ASSESSING ADRS IN CLINICAL PRACTICE
Pharmacists as well as all healthcare professionals should take an active role in monitoring, reporting, and trending ADR information.
PREVENTING AND MONITORING ADR
It was recommended that VERBAL TERMS should be used to describe the risk of experiencing an ADR:
"very common", "very rare", "common"
Another approach is the use of pictures:
Faces, graphs, charts
EXPLAINING RISKS TO PATIENTS