Section 2.3: Modern Atomic Theory and Laws That Led to It
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
Atomic Theory
the idea that all matter is composed of atoms.
Each element is composed of tiny, indestructible particles called atoms.
All atoms of a given element have the same mass and other properties that distinguish them from the atoms of other elements.
Atoms combine in simple, whole-number ratios to form compounds.
Atoms of one element cannot change into atoms of another element. In a chemical reaction, atoms only change the way they are bound together with other atoms.
Law of Conservation of Mass
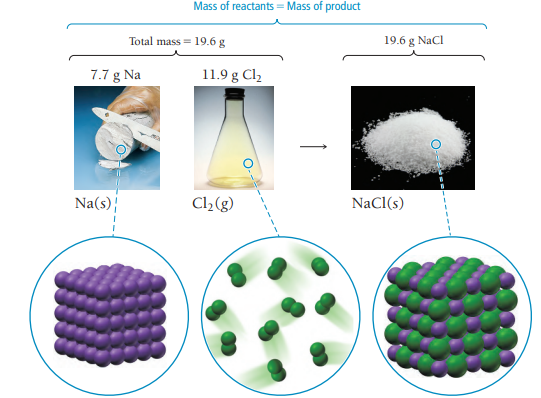
In a chemical reaction, matter is neither created nor destroyed. (When a chemical reaction occurs, the total mass of the substances involved in the reaction does not change. The particles rearrange during a chemical reaction, but the amount of matter is conserved because the particles themselves are indestructible (at least by chemical means). (See image)
Law of Definite Proportions
All samples of a given compound, regardless of their source or how they were prepared, have the same proportions of their constituent elements. EX: In 18.0g of water, there are 16.0g of Oxygen and 2.0g of hydrogen. The Oxygen-to-Hydrogen ratio is 16.0gO/2.0gH = 8.0 or 8:1 ratio. (See image).
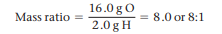
Law of Multiple Proportions
When two elements can combine to make more than one compound, they always do it in simple whole-number ratios. EX: Imagine carbon and oxygen are building with lego blocks. In carbon monoxide (CO): 1 carbon block + 1 oxygen block. In carbon dioxide (CO2): 1 carbon block + 2 oxygen blocks. If you keep the amount of carbon the same, the amount of oxygen that combines with it is in a simple ratio: 1:2. (When the same elements make different compounds, the masses of one element that combine with a fixed mass of the other are in small whole-number ratios (like 1:2, 2:3, 3:4 — not fractions).