Chemistry - Unit 6 Flashcards
5.0(1)
Card Sorting
1/30
Earn XP
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
1
New cards
LAST UNIT OF THE TRIMESTER WE GOT THIS U GUYS
I BELIEEEEEVEEEE
2
New cards
okay, so this unit deals w/ a looot of numbers
so buckle up and get ready for lots of practice, and refer to the resource page too!
3
New cards
back to the basics: tell me, what is the number for a mole of particles?
6.02 hexillion, or 6.02 x 10^23
4
New cards
what is the format of scientific notation?
A x 10^B
5
New cards
what does "B" represent in scientific notation?
the # of decimal places to be moved
6
New cards
what is the purpose of scientific notation?
to reduce the number of zeros in big big number, u don't wanna deal with that
7
New cards
when writing in scientific notation, how many numbers should be in front of the decimal?
one. just one.
8
New cards
alright alright now POP QUIZ: write 93,000,000 in scientific notation. GO!
9.3x10^7
9
New cards
now for our fav thing ever...
sig figs....yay...
10
New cards
let's ask ourselves because we might be asked on the test: what are significant figures, and why do we use them?
- #of digits or figures that give reasonably reliable info
- used to show how precise a # is
what that actually means...idk u tell me
- used to show how precise a # is
what that actually means...idk u tell me
11
New cards
now the thingy about sig figs is their special rules, so let's get into those (flip the card)
I'll give you a rule and you tell me whether it does or doesn't represent a sig fig
12
New cards
nonzero integers, ie: 1,2,3,4, etc.
sig fig!
13
New cards
trailing zeros: zeros @ the right end of the number w/o a decimal point (ie: 2900)
NOT a sig fig
14
New cards
leading zeros: zeros that come before the nonzero digits (ie: 0.000038)
NOT a sig fig, only there for the ~aesthetic~
15
New cards
captive zeros: zero held hostage in between nonzero #'s (ie: 207609)
sig fig!
16
New cards
exact numbers: numbers not obtained by using measuring devices (ie: 1 day= 24 hrs)
NOT a sig fig
17
New cards
trailing zeros: zeros at the right end of a decimal (ie: 3.00)
sig fig!
18
New cards
what is percent composition?
the percentage of each material found in a compound (by mass)
equation: mass of element/total mass x 100
equation: mass of element/total mass x 100
19
New cards
BOO! ANSWER THIS QUESTION: what is the percent composition of nitrogen in copper (II) nitrate?
about 14.94% Nitrogen
20
New cards
now for ur fav: dimensional analysis!!!!!?!?!?!!??!!
i'm so tired
21
New cards
tell me, wth is dimensional analysis?
literally just converting from one unit of measurement to another
22
New cards
easy right?
W R O N G
23
New cards
to get a better understanding of dimensional analysis, tell me, what are conversion factors?
fractions with numerator and denominator of EQUAL value
24
New cards
how do we use conversion factors to get to new units of measurement?
multiply the fractions by putting the unit you want to cancel out on the denominator of the next fraction ie:
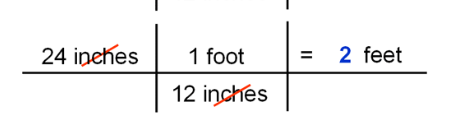
25
New cards
true or false: you can convert from moles,->grams
true
26
New cards
yuh or nuh uh: you can convert from atoms
yuh
27
New cards
yahoo or boohoo: you can convert from grams
BOOHHOO. YOU MUST HAVE MOLES FIRST. WHY? IDK I FORGOT BUT REMEMBER THAT.
28
New cards
now for that new stuff we were taught but weren't rlly taught since she wasn't here to teach it
empIRICal and mOleCular FormULas OmG I loVE thEM
29
New cards
wuts molecular formula?
formula that gives the total number of atoms of each element present in a compound
30
New cards
ok now what's empirical formula?
the teensy tinest ratio of atoms present in a compound, kind of like reducing the subscripts to the smallest amount
31
New cards
OK I THINK THAT'S AB EVERYTHING THAT WORKS W/ FLASHCARDS GO LOOKIE AT THE RESOURCES FOR PRACTICE AND NOTES AND STUFF HAVE A GOOD DAY