Buffers and Titrations
1/25
Earn XP
Description and Tags
from powerpoint
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
what is a buffer?
solutions that resist changes in pH when an acid or base is added - •they act by neutralizing the added acid or base
what does a buffer solution contain?
contains a combination of acid-base conjugate pairs, (means it may contain a weak acid and its conjugate base or a weak base and its conjugate acid )
Example problem: How do you prepare a buffer using HF and NaF? READS: How many moles of NaF must be added to 0.5 L of 0.2 M HF to make a buffer with pH = 3.50?
Ka=6.8×10^-4
How many moles of NaF must be added to 0.5 L of 0.2 M HF to make a buffer with pH = 3.50?
Ka=6.8×10^-4
pKa=-log(Ka)
pKa=3.17
0.2M HF * 0.5L = 0.1M
Solution:
Henderson-Hasselbalch Equation:
pH=pKa+log(f-/HF)
3.50=3.17+log([F−]/0.1)
Solve for [F−]
3.50−3.17=log([F−]/0.1)
0.33=log([F−]/0.1)
10^0.33≈2.14
[[F−]/0.1]=2.14
[F-] = 0.214
0.214 moles = NaF
Describe how to use a salt to make a buffers ( weak acid conjugate pair)
Example: How does adding sodium acetate (CH₃COONa) help create a buffer with acetic acid (CH₃COOH)?
Salt Dissociation:
CH3COONa→CH3COO−+Na+CH₃COONa \to CH₃COO^- + Na^+CH3COONa→CH3COO−+Na+
Increases CH₃COO⁻ (conjugate base) concentration.
Shifts Equilibrium Left:
CH3COOH+H2O⇌CH3COO−+H3O+
More CH₃COO⁻ suppresses acid dissociation (Le Chatelier’s Principle).
Lowers H₃O⁺ concentration, stabilizing pH.
Buffer Effect:
Resists pH changes when acid (H₃O⁺) or base (OH⁻) is added.
Describe how to use a salt to make a buffers ( weak Base conjugate pair)
How does adding ammonium chloride (NH₄Cl) help create a buffer with ammonia (NH₃)?
Salt Dissociation:
NH4Cl→NH4++Cl−NH₄Cl \to NH₄^+ + Cl^-NH4Cl→NH4++Cl−
Increases NH₄⁺ (conjugate acid) concentration.
Shifts Equilibrium Left:
NH3+H2O⇌OH−+NH4+NH₃ + H₂O \rightleftharpoons OH^- + NH₄^+NH3+H2O⇌OH−+NH4+
More NH₄⁺ suppresses base dissociation (Le Chatelier’s Principle).
Lowers OH⁻ concentration, stabilizing pH.
Buffer Effect:
Resists pH changes when acid (H₃O⁺) or base (OH⁻) is added.
How to make a buffer through strong/base neutralization or adjust the ratio of the conjugate of the acid / base pair
How can you create a buffer using HF and a strong base?
Add a Strong Base (e.g., NaOH) to HF Solution:
NaOH neutralizes some HF, forming F⁻ (its conjugate base).
HF+OH−→F−+H2O
Adjust the Ratio of HF to F⁻:
Use the Henderson-Hasselbalch equation to set the desired pH:
pH=pKa+log([F−][HF])
Adding more NaOH increases F⁻, shifting pH up.
Adding more HF increases HF, shifting pH down.
Result:
The HF/F⁻ system now acts as a buffer, resisting pH changes.
Which combination when mixed makes a buffer solution(assume large amounts of reactant are left over
A. HCl and KCl
B. H2CO3 and NaHCO3
C. H3PO4 and NaCl
D.CH3COOH and CH3COOK
E.NH3 and NH4Cl
✅ B. H₂CO₃ and NaHCO₃ (carbonic acid & bicarbonate, weak acid/conjugate base)
✅ D. CH₃COOH and CH₃COOK (acetic acid & acetate, weak acid/conjugate base)
✅ E. NH₃ and NH₄Cl (ammonia & ammonium, weak base/conjugate acid)
Why Not the Others?
❌ A. HCl and KCl – HCl is a strong acid, no buffer.
❌ C. H₃PO₄ and NaCl – NaCl does not provide a conjugate base.
Which combination makes a buffer solution with the use of salts? ( Assume large amounts of everything )
A. HI and NaOH
B. H2CO3 and NaOH
C.NH3 and NaOH
D.NH3 and HNO3
HF + LiOH
The combinations that form buffer solutions using salts are:
✅ B. H₂CO₃ and NaOH → Forms HCO₃⁻ (bicarbonate), creating a H₂CO₃/HCO₃⁻ buffer
✅ E. HF and LiOH → Forms F⁻, creating an HF/F⁻ buffer
Why Not the Others?
❌ A. HI and NaOH → HI is a strong acid, no buffer.
❌ C. NH₃ and NaOH → Both are bases; no weak acid to form a buffer.
❌ D. NH₃ and HNO₃ → Strong acid fully reacts with NH₃, leaving no weak base/conjugate
The addition of hydrofluoric acid and _____ to water produces a buffer solution
A. NaBr
HCL
NaNO3
NaOH
NaCl
NaOH
Explanation:
HF is a weak acid, and NaOH is a strong base.
When NaOH is added to HF, it neutralizes part of the HF, forming F⁻ (fluoride ion), which is the conjugate base of HF.
This creates a HF/F⁻ buffer system that can resist pH changes.
Why Not the Others?
❌ NaBr, HCl, NaNO₃, NaCl – None of these form a buffer solution with HF because they do not provide a conjugate base or acid.
which one of the following pairs cannot be mixed together to form a buffer solution
A. NaCL, HCL
b. HONH2, HONH3Cl
c. KOH, HNO2
d. H2SO3, KHSO3
E. RbOH, HF
The pair that cannot form a buffer solution is:
❌ A. NaCl, HCl
Explanation:
NaCl is a neutral salt, and HCl is a strong acid. A buffer requires a weak acid and its conjugate base (or a weak base and its conjugate acid). HCl dissociates completely in water and does not provide a conjugate base to form a buffer.
Why the Others Work:
B. HONH₂ and HONH₃Cl: HONH₂ (hydroxylamine) is a weak base, and HONH₃Cl is its conjugate acid, forming a buffer.
C. KOH and HNO₂: KOH (strong base) neutralizes some of the HNO₂ (weak acid), creating the conjugate base NO₂⁻, forming a buffer.
D. H₂SO₃ and KHSO₃: H₂SO₃ (weak acid) and HSO₃⁻ (its conjugate base) form a buffer system.
E. RbOH and HF: RbOH (strong base) neutralizes part of HF (weak acid), creating the conjugate base F⁻, forming a buffer.
What is the pH of a buffer that is 0.100 M HC2H3O2 and 0.100 M NaC2H3O2?
Ka for HC2H3O2 = 1.8 x 10-5
HC2H3O2 + H2O ⇋ C2H3O2- + H3O+
Step 1: Calculate pKa pKa=−log(1.8×10−5)≈4.74\
Step 2: Apply the Henderson-Hasselbalch equation
Given:
[A⁻] = 0.100 M (from NaC₂H₃O₂)
[HA] = 0.100 M (from HC₂H₃O₂)
pH=4.74+log(0.100/.100)
pH=4.74+log(0.100/0.100)
pH=4.74+log(1)
ph = 4.74 + 0
The pH of the buffer solution is 4.74.
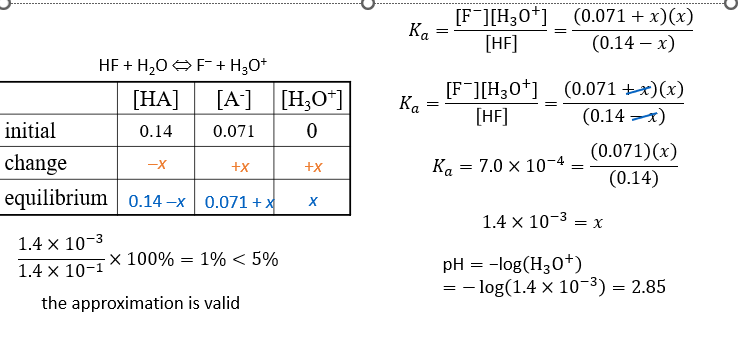
Practice - What is the pH of a buffer that is 0.14 M HF and 0.071 M KF? Ka for HF = 7.0 x 10-4
Ice table and Ka calculation - see image for example
What is the final pH after adding 0.010 moles of HCl to 500.0 ml of a 0.125 M HC2H3O2 and 0.115 M NaC2H3O2 buffer system.
Step 1: Write the balanced equation for the buffer system
The buffer consists of HC₂H₃O₂ (acetic acid) and its conjugate base C₂H₃O₂⁻ (acetate ion). The reaction between acetic acid and the strong acid HCl is:
HC₂H₃O₂+HCl→C₂H₃O₂−+H₂O
Step 2: Determine the initial moles of acid and base
Given:
Volume of the buffer solution = 500.0 mL = 0.500 L
0.125 M HC₂H₃O₂ (acetic acid) and 0.115 M NaC₂H₃O₂ (acetate ion).
Initial moles:
HC₂H₃O₂ (acetic acid):
moles of HC₂H₃O₂=0.125 M×0.500 L=0.0625 moles
C₂H₃O₂⁻ (acetate ion):
moles of C₂H₃O₂⁻=0.115 M×0.500 L=0.0575 moles
Step 3: Add the strong acid (HCl)
You are adding 0.010 moles of HCl to the buffer system.
HCl dissociates completely, so it adds 0.010 moles of H⁺ to the solution.
The added H⁺ will react with C₂H₃O₂⁻ (the conjugate base) to form more HC₂H₃O₂ (acetic acid).
Step 4: Adjust the moles of acid and base after the neutralization
C₂H₃O₂⁻ (conjugate base) will decrease by 0.010 moles because it reacts with the H⁺ from HCl.
HC₂H₃O₂ (acetic acid) will increase by 0.010 moles.
So the final moles are:
HC₂H₃O₂:
0.0625 moles+0.010 moles=0.0725
C₂H₃O₂⁻:
0.0575 moles−0.010 moles=0.0475 moles
Step 5: Use the Henderson-Hasselbalch equation
Now, we can apply the Henderson-Hasselbalch equation to find the final pH:
pH=pKa+log([C₂H₃O₂−][HC₂H₃O₂])
First, calculate the new concentrations in the 500.0 mL (0.500 L) solution:
[HC₂H₃O₂]:
0.0725 moles0.500 L=0.145 M
[C₂H₃O₂⁻]:
0.0475 moles0.500 L=0.095 M
Step 6: Calculate the pH
pKa for acetic acid (HC₂H₃O₂) is 4.74.
pH=4.74+log(0.0950)(.145)]
pH=4.74+log(0.655)
pH=4.74+(−0.183) pH=4.56
pH=4.56 Answer:
The final pH of the buffer after adding 0.010 moles of HCl is 4.56.
Calculate the pH of a buffer that is 0.225 M HC2H3O2 and 0.162 M KC2H3O2. The Ka for HC2H3O2 is 1.8 × 10-5.
pH=pKa+log([HA][A−])
Where:
[A⁻] is the concentration of the conjugate base (acetate ion, C₂H₃O₂⁻, from KC₂H₃O₂).
[HA] is the concentration of the weak acid (HC₂H₃O₂).
pKa is the negative logarithm of Ka.
Step 2: Calculate pKa
Given Ka for acetic acid (HC₂H₃O₂) is 1.8 × 10⁻⁵:
pKa=−log(1.8×10−5)≈4.74
pKa=−log(1.8×10−5)≈4.74
Step 3: Plug Values into the Henderson-Hasselbalch Equation
Given concentrations:
[HA] = 0.225 M (from HC₂H₃O₂)
[A⁻] = 0.162 M (from KC₂H₃O₂)
Now apply the values:
pH=4.74+log(0.1620.225)
pH=4.74+log(0.2250.162)
pH=4.74+log(0.72)
ph = 4.74 + (-0.143)
pH = 4.60
Compare the effect on pH of adding 0.010 mol NaOH to a buffer that has 0.100 mol HC2H3O2 and 0.100 mol NaC2H3O2 in 1.00 L to adding 0.010 mol NaOH to 1.00 L of pure water?
See image,
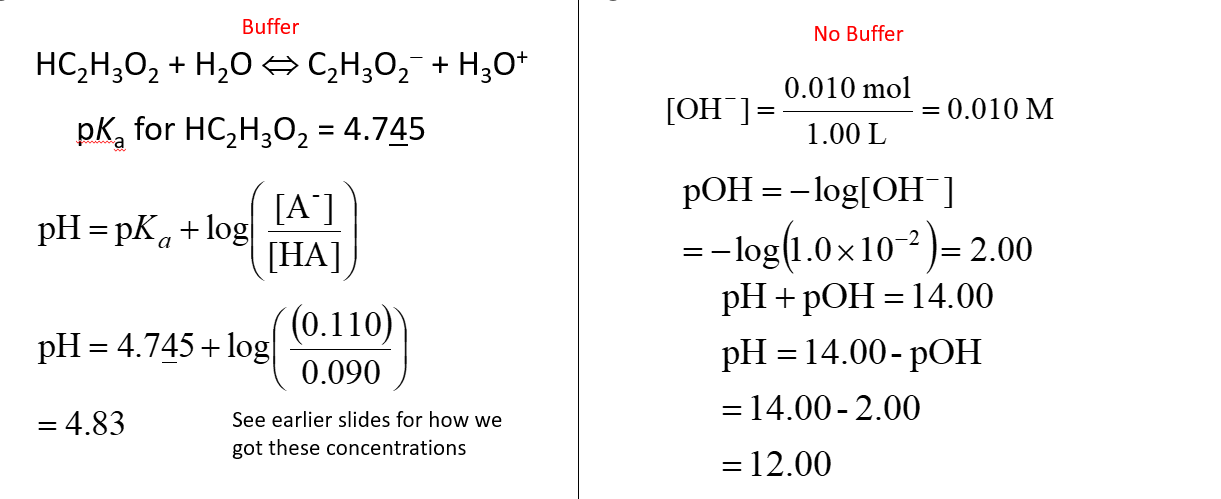
what is buffering range?
the pH Range that the buffer can be effective.
what is the buffering capacity
the amount of acid or base a buffer can neutralize
Which of the following acids would be the best choice to combine with its sodium salt to make a buffer with pH 4.25?
chlorous acid pKa=1.95
nitrous acid pKa=3.34
formic acid pKa=3.74
hypochlorous acid pKa=7.54
To determine which acid would be the best choice for creating a buffer with a pH of 4.25, we need to refer to the Henderson-Hasselbalch equation:
pH=pKa+log([A−][HA])
Where:
pKa is the acid dissociation constant of the acid.
[A⁻] is the concentration of the conjugate base (the salt form of the acid).
[HA] is the concentration of the weak acid.
For a buffer, the pKa of the acid should be close to the desired pH. Ideally, the pKa should be within 1 unit of the target pH, as the buffer works best when the pH is near the pKa.
The best choice to create a buffer with pH 4.25 is formic acid (pKa = 3.74). It is the acid with the pKa closest to 4.25, making it the most suitable for this purpose.
what is the solution of unknown concentration in titrations known as?
titrant
when is a titration complete?
when the moles of H3O+=OH-
what is the difference between solving titrations between buffer problems and ice problems
Key Differences:
Approach:
Buffer Problems: Focuses on the resistance to pH change in a solution containing a weak acid and its conjugate base, often using the Henderson-Hasselbalch equation.
ICE Problems: Focuses on calculating equilibrium concentrations and pH after reactions (such as acid dissociation or neutralization) based on initial concentrations and equilibrium constants.
Reaction Type:
Buffer Problems: You are primarily concerned with how the buffer components interact with the added acid/base to maintain a relatively constant pH.
ICE Problems: You are solving for equilibrium concentrations of species in a dissociation or neutralization reaction (often during a titration) and may involve the full stoichiometry of the neutralization process.
pH Calculation:
Buffer Problems: Use the Henderson-Hasselbalch equation to calculate pH after adding acid or base.
ICE Problems: Set up an ICE table to determine equilibrium concentrations and then use those to calculate pH based on either [H⁺] (for acids) or [OH⁻] (for bases).
In Titration:
Buffer: You calculate pH changes as a result of acid/base addition to a buffer solution using the Henderson-Hasselbalch equation.
ICE: You calculate pH changes during a titration (such as titrating a weak acid with a strong base) using stoichiometry and equilibrium constants to find the concentrations of acid/base at different stages of the titration.
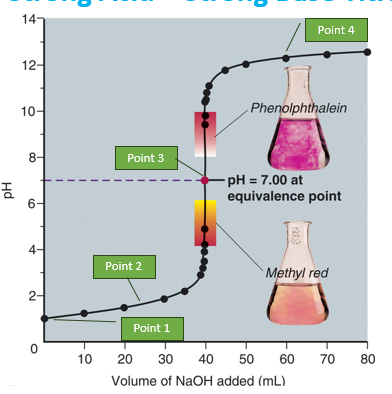
strong acid + strong base titration graph
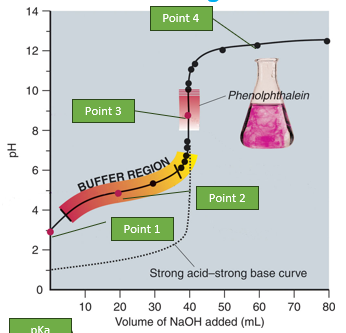
weak acid and strong base titration graph
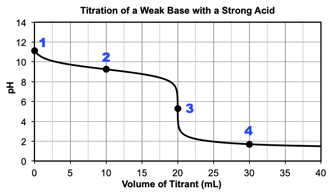
weak base plus strong acid titration graph
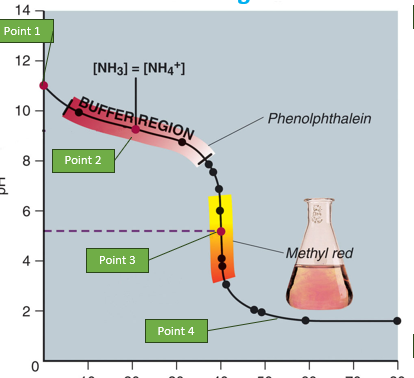
based on the graph were is the buffer region
Titration of a weak base with a strong acid
2
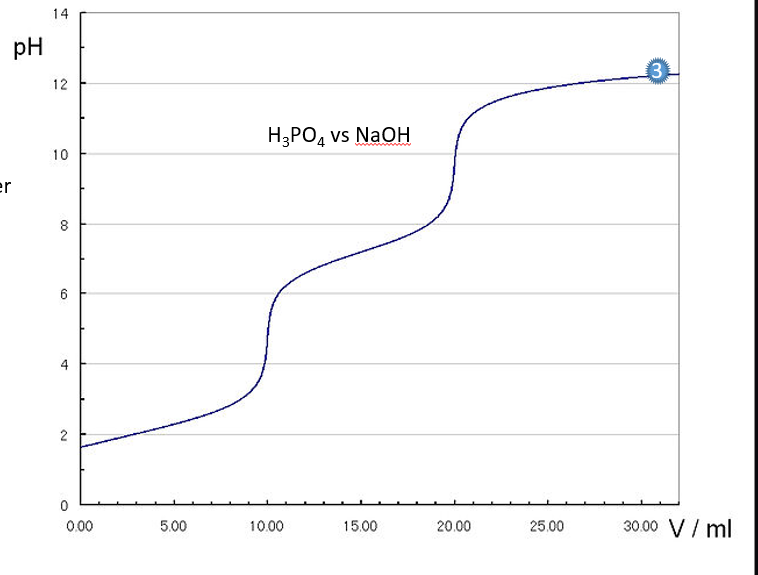
Estimate
•Equivalence point 1
•Equivalence point 2
•Equivalence point 3
•pKa1,pKa2,pKa3
•Ka1,Ka2,Ka3
Label or Write
•Buffer region
Summary of Key Points:
Equivalence Points: Found at the steep vertical regions of the titration curve (pH jumps). You can estimate the mL of NaOH added at these points.
Buffer Regions: Found between equivalence points, characterized by gradual pH changes. The pKa values are roughly in the middle of these regions.
pKa Values: Estimated from the midpoints of buffer regions.
Ka Values: Calculated as Ka=10−pKa\text{Ka} = 10^{-\text{pKa}}Ka=10−pKa.
Example:
If the graph shows the following pH jumps:
First equivalence point at 10 mL, pH ~ 5.
Second equivalence point at 20 mL, pH ~ 9.
Third equivalence point at 30 mL, pH ~ 12.
Then:
pKa1 is around pH 2.5–3.5 (halfway between 0 and 10 mL).
pKa2 is around pH 7.5–8.5 (halfway between 10 and 20 mL).
pKa3 is around pH 11 (halfway between 20 and 30 mL).
Finally, you can calculate the Ka values as:
Ka1 ≈ =}10^−pKa1
Ka2 ≈ 10^−pKa2
Ka3 ≈ =10^−pKa3