AST101 - Midterm 2
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
Scattering
Reflection of all the colours in all directions
colour you see is ______
Absorption
for black objects, all colours of light are _____
for coloured objects, the colour you see is scattered, all other colours are ____
Emission
the process where an atom or molecule releases energy as a photon (a particle of light) when an electron moves from a higher to a lower energy level
Transmission
the passage of light through a material, like a planet's atmosphere
Screens
screens work though emission
White: all lights on
Black: all lights off
yellow: Red and Green on
Cyan: Green and blue on
Magenta: Blue and red on
Wavelength
distance between peaks
amplitude
hight of peaks
frequency
how often peaks arrive
speed of a wave
s=fL
at a constant speed:
shorter wavelengths means higher frequency
longer wavelengths means lower frequency
light
light is an electromagnetic wave
all light travels at 300,000 km/s
the wavelength (or frequency) determines colour

thermal radiation
anything that is ‘warm’ glows
the temperature of the object effects its spectrum
hot things are brighter (per area)
the spectrum of hot things peaks at shorter wavelength
warmer: shorter wavelength
cooler: longer wavelength
molecular clouds are very cold (-260C or 13K)
so they glow with very very long wavelength light
called “sub-mm light”
not visible to the eye
electromagnetic spectrum (left to right)
gamma rays
x-rays
ultraviolet
visible
infrared
radio
hot gas emission
when low pressure gas is excited electrically, it glows with distinct colours
much of the light comes out in specific colours called emission lines
each element has its own patter
this is how old neon signs worked
quantum mechanics
the electron around a hydrogen atom can be exactly in one of many possible cloud configurations
each cloud has a different energy
each type of atom has different set of clouds
bohr atom (quantum mechanics simplified)
draw each orbital as a simple circle
bigger circles have higher energy
when an electron shifts between energy levels, it gives off light go frequency
f=ΔE/h
energy levels
a specific amount of energy a particle, like an electron, can have within an atom or other quantum mechanical system
depending on the arrangement, you can get either emission lines or absorption lines
bohr atom: absorption
consider white light shining on an atom
light that is exactly the right colour (f=ΔE/h) will be absorbed, and the electron will go to a higher orbital
the other colours will pass right by
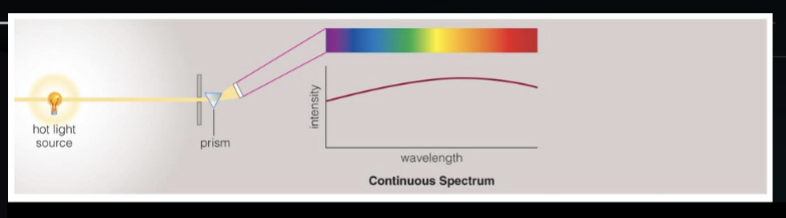
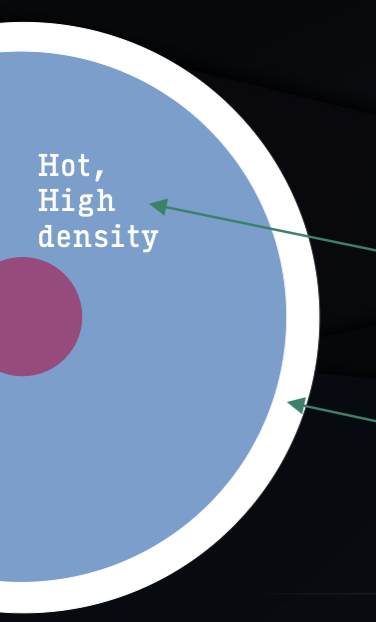
thermal radiation from a hot dense source
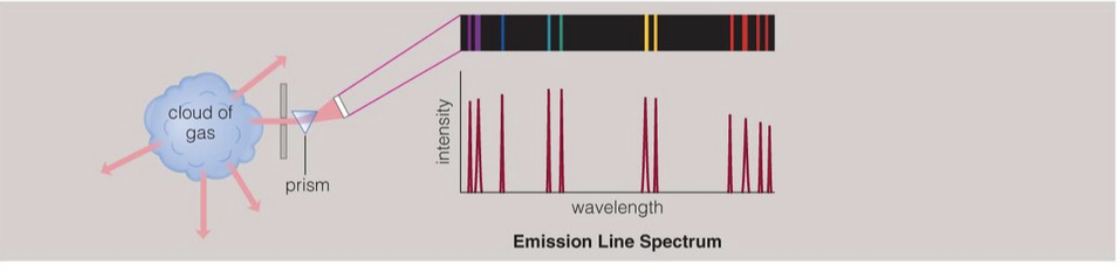
emission line spectra from hot low density gas
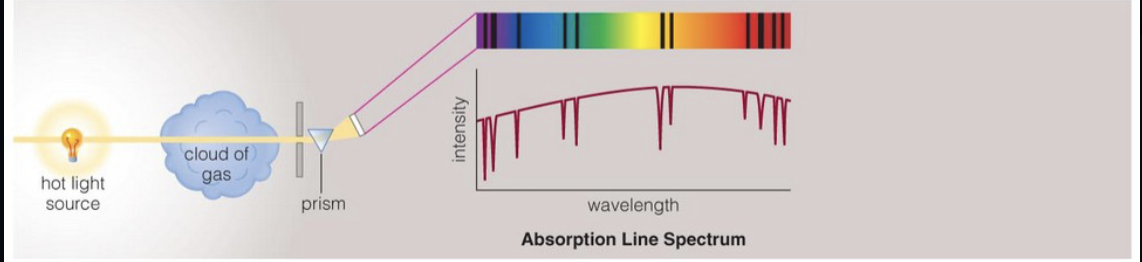
absorption line spectra from cool low density gas with a hotter source behind it
understanding the sun’s spectrum
the sun’s spectrum is continuum plus absorption lines
continuum is due to opaque, high density hot interior (blackbody)
absorption lines are due to cooler gas in the photosphere
types of spectra
the difference in energy levels in the same for both emission and absorption
the colour (frequency) is the same for both emission and absorption
both emission and absorption spectra can be used as chemical fingerprints
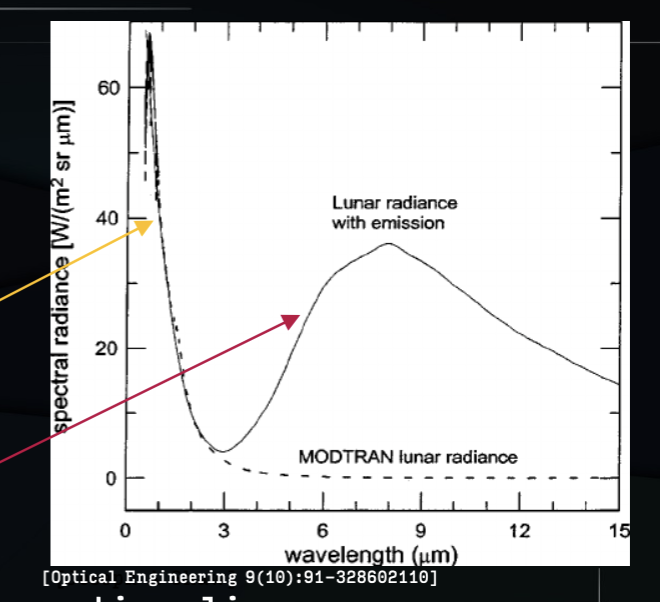
the moon’s spectra
2 parts:
reflection of the sun’s thermal spectrum
thermal emission from the warm rock
no atmosphere, so no absorption lines
Nebula
nebula produce emission lines
these lines can be used to determine the cloud’s composition
absorption spectra from gas clouds in space
light from star travels through the clouds
absorption line can be used to determine cloud content
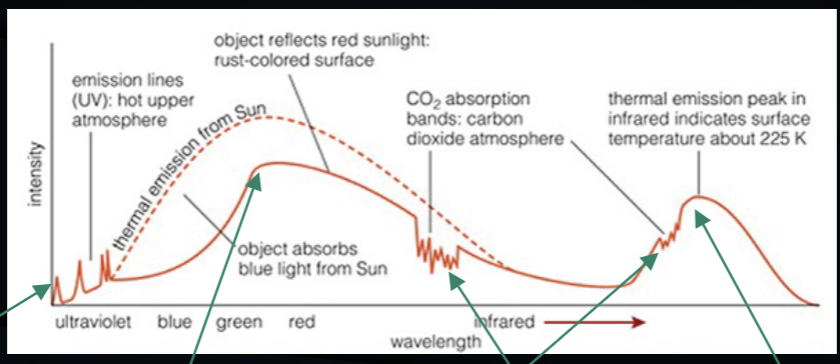
the spectrum of mars from space
hot gas above the main atmosphere
sun’s spectrum is scattered- but the red rock absorbs blue light
the CO2 atmosphere produces absorption lines
mars is around 255K, so it produces a 255K thermal spectrum
source of light
temperature:
the location of the peak of the thermal spectra
emission and absorption lines
Density:
thermal spectra vs emission/absorption lines
the width of the lines
composition:
each atom type has unique lines
doppler effect
the change in the frequency of a wave (like sound or light) due to the relative motion between a source and an observer.
sound is a pressure wave
pitch is the frequency
when the source of a wave is coming towards you, the pitch is higher
when the source of a wave is going away from you, the pitch is lower
same happens with light
if the source is moving closer, the light shifts higher frequency (i.e. it is “blue-shifted”)
if the source is moving away, the light shifts to lower frequency (i.e. it is “red-shifted”)
molecular clouds
in galaxies
mix of gas and dust
~3/4 hydrogen
~1/4 helium
traces of other elements (including carbon dust)
appear black (because the dust blocks light)
pressure, temperature, and density
Gas and plasma are made of particles
these particles are moving
when they bounce off something, they apply pressure
higher temperature:
moving faster
higher pressure
higher density
more particles
higher pressure
P=kTN/V
parts of the sun
core:
fusion takes place here
Around 10 million C
over 100x denser of water
Radiative zone:
hot but calm
a few million C (no fusion)
around the density of water
convection zone:
hot plasma rises, brining heat to surface
hundred of thousands of C
density of styrofoam
hydrostatic equilibrium in a star - density above
density ___ ‘equilibrium’ → rate of fusion increases → temperature increases → pressure increases → core expands → density drops back to equilibrium → equilibrium restored
hydrostatic equilibrium in a star - density below
density ___ ‘equilibrium’ → rate of fusion decreases → temperature decreases → pressure decreases → core contracts → density increase back to equilibrium → equilibrium restored
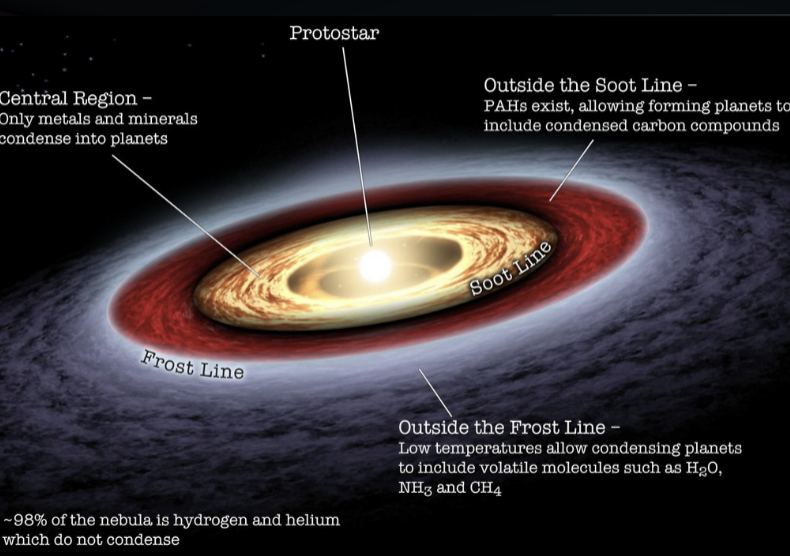
Nebular Hypothesis
the widely accepted model for how solar systems form, proposing that they originate from a large, rotating cloud of gas and dust called a solar nebula
planet formation
the force of gravity pulls a molecular cloud (made of gas and dust) together
as it collapses, it beings to spin faster (conservation of momentum)
collisions between particles flatten the orbit
forming a spinning disk of gas and dust
small bits of material stick to larger bits of material
eventually, gravity pulls them together to form planets
frost line
the closer to the protostar, the hotter it is
the distant disk is cold enough for light molecules to condense/freeze
in the near disk, only heavier molecules like metal can condense
the disk is 98% hydrogen and helium
it is only 2% everything else
outside the frost line, most of everything else can condense
inside the frost line, only metals can- this is a lot less
terrestrial planets form inside the first line
jovian planets form outside the frost line
the heavy bombardment period
early on, there were very large numbers of planetesimals (asteroids and comets)
collisions were frequent
the surfaces of airless bodies like the moon show the outcome of this period
there are far fewer now
ended about 4 billion years ago
where did the moon come from
rocks show that the surface of the moon is made from the same material as the earth’s curst
the moon was too large to have been formed with the earth
according to newton’s laws, the moon could not have been captured either
of it wasn’t already in orbit, it should have just flown on by
creating a crater
a planetesimal strikes the surface
typical speed is 100,000 km/hr
the collision vaporizes the rock and creates an enormous explosion
a crater is left
types of vibrations
P waves - compression
S waves - Side to side
planet interior
curst → low density rock
mantle → medium density rock
core → highest density, iron and nickel
how do planets cool?
step 1: convection
a planet’s mantle is not completely rigid
hot rock weighs less than cooler rock
the hot rock rises and cooler rock sinks
the brings heat up from the core
step 2: conduction
a planet’s cool crust is ridgid, so there is no convection there
heat is conducted through the rock (slower than convection)
crust is very thin
Step 3: Radiation
infrared light carries energy away from the surface of the planet
if there is more light leaving the planet than coming from the sun, the planet cools
step 4: cooling- does planet size matter?
volume = 4/3πr3
area = 4πr2
if you double the radius
the volume is 8 times bigger than area is 4 times bigger
small planets have less mass compares to it’s surface area than a large planet
small planets cool faster
blackbody spectrum
continuous graph showing the intensity of light an object emits at different wavelengths, based only on its temperature.
craters on earth
meteor crater in Arizona
nickel-iron meteorite
50 meters across
47,000 km/hr impact speed
50,000 years ago
there are 190 impact craters on earth
most of them barely visible
most are fairly recent
all but the very youngest show signs of very significant erosion
the oldest ones are extremely large
none are from the heavy bombardment period
volcanism
earths moon:
small
cooled fast
volcanism ended quickly
no atmosphere
craters mostly not erased
mars:
larger than the moon
cooled later
thin atmosphere
early craters erased
venus:
2nd largest terrestrial planet
still cooling
extremely low winds
most craters erased by volcanism
earth:
largest terrestrial planet
still cooling
winds and water
most craters erased
plate tectonics
subduction at converging plates
ocean plate gets pushed under continental plate
continental plate gets pushed up into mountains
diverging plates:
causes undersea ridges
astronomy chemistry
atmospheres are mainly made from oxygen, nitrogen, carbon, and hydrogen
oxygen is 16 times as heavy as hydrogen
escape velocity and molecular mass
gas is made of particles, they are all moving
higher temperature:
faster average speed
higher mass
slower average speed
objects going faster than a planet’s escape velocity will escape into space
lower mass planets have lower escape velocity
it is easier to escape a small planet’s gravity
low mass planet
escape velocity lower
easier to escape
low mass gas
moves faster
easier to escape
hot gas
moves faster
easier to escape
earths atmosphere: the carbon cycle
CO2 in the air dissolves in water, then forms carbonate rocks
most of the CO2 that was in the atmosphere is now in carbonate rock
N2 is left as the dominate gas
radiative heating
visible light form the sun shines on the planet
some is reflected and the rest is absorbed
infrared light is emitted by the planet
the warmer the planet, the more infrared light is emitted
the planet warms until absorption of visible light and emission of infrared light are balanced
greenhouse effect
visible light passes through the atmosphere
some visible light is reflected, the rest is absorbed
the surface emits infrared radiation because it is warm
greenhouse gasses absorb and remit the infrared radiation, acting like a blanket, warming the surface
the top of the atmosphere is cool as expected
why isn’t earth colder
given the reflectance of earth, and its distance from the sun, earth should have an average temperature of -16C
on the surface it is actually warmer 15C
this is from the greenhouse effect, from water vapour, CO2 and CH4
water vapour is the most important
climate change
temperature on earth varies with CO2
low CO2 levels lead to ice ages
high CO2 levels lead to warmer periods
the current rapid rise in CO2 from burning fossil fuels will increase surface temperatures more than we have seen in millions of years
photodissociation
a chemical reaction where a molecule breaks down after absorbing light energy,
UV light from the sun breaks apart atoms
the individual atoms are lighter than the molecule, so they can escape easier
they will reconnect as soon as they find each other again
photosynthesis
plants convert water and carbon dioxide into oxygen and carbohydrates
this produces the oxygen in atmosphere
burning is the reverse
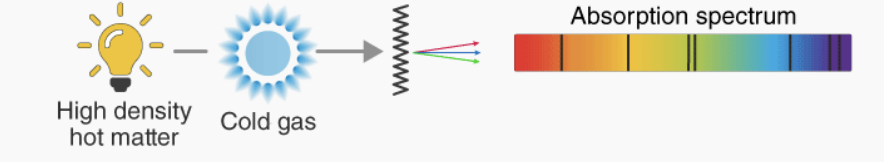
Absorption lines
dark lines in a spectrum that appear when a cooler gas absorbs specific wavelengths of light from a hotter background source
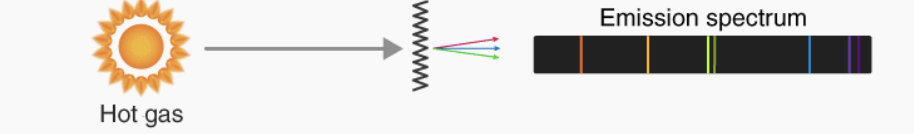
Emission lines
specific, narrow bands of light observed in the spectrum of an object, created when electrons in excited atoms drop to lower energy levels, releasing photons of a discrete energy and wavelength