Unit 1 Exam: Water and Biological Molecules (copy)
1/40
Earn XP
Description and Tags
Biology
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
A molecule that has a partial positive charge on one side and a partial negative charge on the other side is called a _______________.
Polar molecule
Similar substance interact with each other, because ________ and __________. select two.
They have similar properties
They have cohesive properties
Carbohydrates contain all of the following except
nitrogen
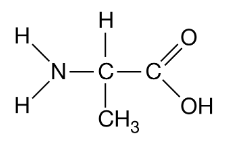
Figure 1. A biological molecule monomer
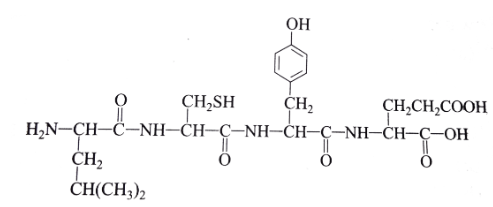
The above monomer in Figure 1 is found in a region of a polypeptide. Which of the following form the basic structure of a biological molecule in figure 1? select two.
The amino (NH2) group
The carboxyl (COOH) group
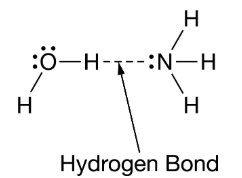
Water and ammonia interact to form hydrogen bonds, as represented in the figure. Which statement best helps explain the formation of the hydrogen bond represented in the figure? Select two.
The greater electronegativity of oxygen and nitrogen compared with hydrogen makes for unequal sharing of electrons, which results in partial negative charges associated with the hydrogen atoms in both molecules, The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge
Which of the following best describes the function of proteins?
Proteins can function as an enzyme
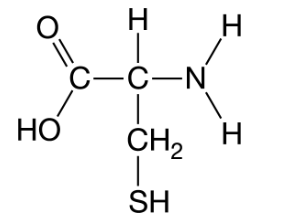
Researchers compared similar proteins from related organisms in different habitats. They found that the proteins from organisms living in harsh environments had a greater number of cysteine amino acids and are more adaptable to the environment than did proteins from organisms not living in harsh environments. The structure of cysteine is shown. Bonds can form between the sulfur atom of a different cysteine amino acids (S-S bonds) making the protein stronger. Figure 1. Chemical structure of cysteine
Which of the following describes the effect of a greater number of cysteine amino acids on the stability of the proteins?
The change leads to increased protein stability making the organism more adaptable to the harsh environment
Upon chemical analysis, a particular molecule was found to contain ___ carbon, 18 hydrogen, 9 oxygen. How many carbons are present in this molecule?
9
Which of the following is not the function of carbohydrates?
enzyme
Which of the following is are/not common to biological molecules? select two.
phosphorus, nitrogen
Which of the followings are properties related to hydrogen bonding? Select three.
adhesion, surface tension, cohesion
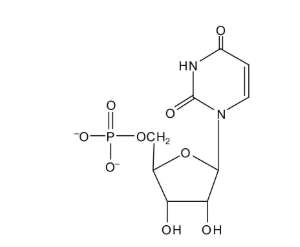
Which of the following is/are example of nucleic acids? Select two
ribose nucleic acid, deoxyribose nucleic acid
Which of the following are not a monomer of biological molecules? Select two.
hemoglobin, steroid
All of the followings are found in proteins except ________.
phosphorus
Which of the following explain the pattern of temperature shown in the diagram above? Select two.
The ability of water to stabilize temperature because of its relatively high specific heat, Large body of water can absorb and store large amount of heat due to the many hydrogen bonds
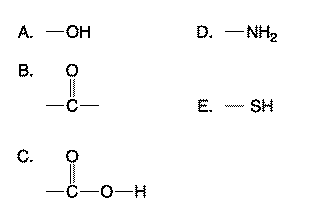
Which functional groups help form peptide bond between amino acids? select two
D, C
What determines the cohesiveness of water molecules? Select two.
hydrogen bonds, polarity of water
Based on the structure above, which of the following is true?
It is a protein
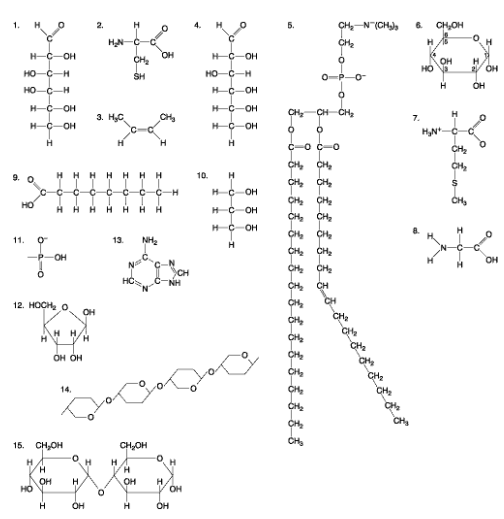
Which of the following molecules are carbohydrates? select two
1, 4
Which of the following differentiate lipids from carbohydrates? select two
Lipids have no distinctive C:H:O ratio, Lipids are nonpolar and therefore cannot dissolve in water, because water is a polar molecule
A biology student conducting an experiment on the effect of fertilizer on strawberry growth. The student decided to use Miracle-Gro as the main source of fertilizer. 'Miracle-Gro' is a 15-30-15 fertilizer, meaning that that the powder within contains 15 percent nitrogen, 30 percent phosphorous, and 15 percent potassium. Which of the following statements is/are true? Select two.
Nitrogen will be incorporated into proteins, Phosphorus will be incorporated into lipids
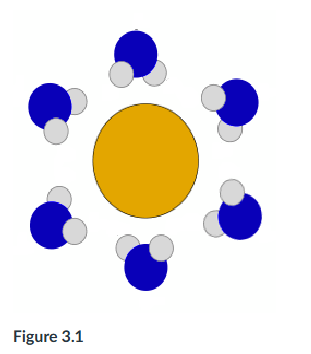
The following question is based on Figure 3.1: solute molecule surrounded by a hydration shell of water. Based on your knowledge of the polarity of water molecules, the solute molecule is most likely ___. Select two.
negatively charged, polar
A molecule with the chemical formula C16H32O16 is probably a ______________.
carbohydrate
The carbohydrates glucose, galactose, and fructose have the same chemical formula (C6H12O6) but different structural formulas, as represented in the figure. Which of the following statements about glucose, galactose, and fructose is most likely true? Select two.
Compounds that have the same chemical formula but different structural formulas usually have different properties, The carbohydrates have different properties because they have different arrangements of carbon, hydrogen, and oxygen atoms
Which of the following characteristics would be used to identify the structure above? Select two
the presence of phosphorus, the presence of nitrogen
Which of the followings are properties related to hydrogen bonding? Select three.
surface tension, cohesion, adhesion
Ice is lighter and floats in water because it is a crystalline structure in which each water molecule is bonded to a maximum of four other water molecules due to ____.
hydrogen bonds
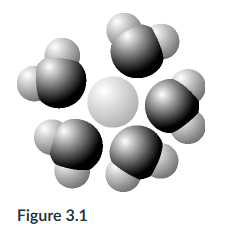
The following question is based on Figure 3.1: solute molecule surrounded by a hydration shell of water. Based on your knowledge of the polarity of water molecules, the solute molecule is most likely ___.
positively charged.
Why does ice float in liquid water? Select two
Hydrogen bonds stabilize and keep the molecules of ice farther apart than the water molecules of liquid water, The crystalline lattice of ice causes it to be less dense than liquid water
An example of a hydrogen bond is the bond between
the H of one water molecule and the O of another water molecule
Which of the following explain the pattern of temperature shown in the diagram above? Select two.
The ability of water to stabilize temperature because of its relatively high specific heat, Large body of water can absorb and store large amount of heat due to the many hydrogen bonds.
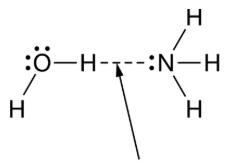
Water and ammonia interact to form a bond, as represented in the figure.
Which statement best helps explain the formation of the bond represented in the figure?
The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge forming a hydrogen bond
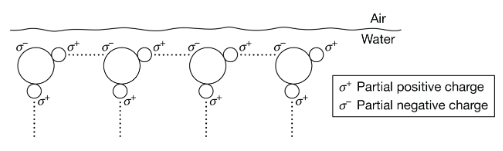
Figure 1 is a diagram of water molecules at the air-water interface at the surface of a pond.
Figure 1. Alignment of water molecules at air-water interface
Based on Figure 1, which of the following describes how the properties of water at an air-water interface enable an insect to walk on the water's surface?
Hydrogen bonds form between water molecules due to the partial charges produced as a result of the polarity of the water molecule. The strength of the attraction between the water molecules allows organisms to walk across the surface without breaking the hydrogen bonds
Which of the following is responsible for water’s unique properties? select two
It forms hydrogen bonds, it is a polar molecule
What results from a unequal sharing of electrons between atoms?
a polar covalent bond
A polar covalent bond can form when ______. Select two
one of the atoms has a greater affinity for electrons than the other atom of the same molecule, two atoms of a molecule attract electrons unevenly
The slight negative charge at one end of one water molecule is attracted to the slight positive charge of another water molecule. What is this attraction called?
a hydrogen bond
What determines the cohesiveness of water molecules? Select two.
hydrogen bonds, polarity of water
Water and ammonia interact to form hydrogen bonds, as represented in the figure.
Which statement best helps explain the formation of the hydrogen bond represented in the figure?
The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.
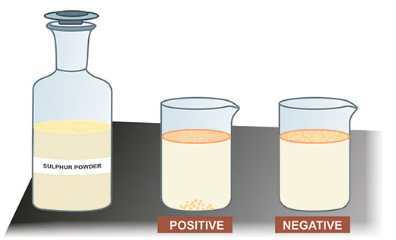
A common test for liver damage involves sprinkling sulfur powder onto a sample of urine. Sulfur powder sprinkled on a healthy individual with normal liver function will float (negative result), while the sulfur powder sprinkled on urine sample of individual with unhealthy liver function will sink (positive result). See figure below. Which of the following best explain why the sulfur powder sink?
Substance in the urine decreases the surface tension of the urine sample.
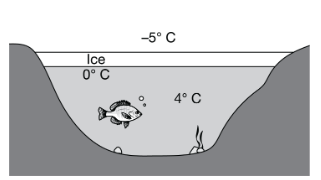
As shown in the diagram, when environmental temperatures drop below freezing, a layer of ice typically forms on the surface of bodies of freshwater such as lakes and rivers.
Which of the following best describes how the structure of ice benefits the organisms that live in the water below?
The water molecules in ice are farther apart than those in liquid water, so the ice floats, maintaining the warmer, denser water at the lake bottom.