DNA/RNA Inhibitors- KEARNS - EXAM 4
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
Match the class to its MOA:
MOA | Names |
DNA Synthesis Inhibitors | |
Folic acid synthesis inhibitors | |
RNA synthesis inhibitors |
Names: Fluoroquinolones, Nitroimidazole, Sulfonamides, Rifamycin
MOA | Names |
DNA Synthesis Inhibitors |
|
Folic acid synthesis inhibitors | sulfonamides |
RNA synthesis inhibitors | rifamycins |
Fluoroquinolones MOA:
Where in the cell do they act?
What phase of DNA synthesis?
What enzymes do they inhibit?
act in the NUCLEUS
S phase of DNA synthesis
INHIBIT Top II (DNA gyrase) and Top IV
What’s the difference between topoisomerases in humans and in bacteria?
bacteria- Top II (aka DNA gyrase) and Top IV
Top II—> supports new double-stranded DNA being elongated by RNA-polymerase or by helicase in a replication fork
Top IV—> segregates daughter chromosomes during the terminal stage of DNA replication
humans- Top I and Top II
Top I—> single strand breaks—> relax supercoils
Top II—> double strand breaks—> untangles DNA
In general what do topoisomerase enzymes reduce?
topological and torsional strain of replicated DNA
What is the pharmacophore of Fluoroquinolones? What carbon is this group on?
MUST be on C3
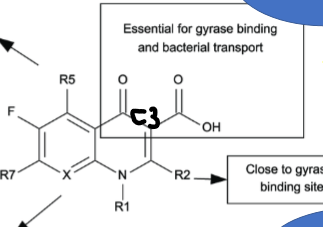
Explain what each R group on fluoroquinolones does:
R1
R5
R7
C8
R1—> determines potency and dosing profile
R5—> determines cell wall penetration
R7—> determines G+ or G- bacterial susceptibility
C8—> controls ANAEROBE activity and some PK
Recognize the structure of EACH fluroquinolone:
PRACTICE:
If I placed a hydrophilic group on R1 of a fluoroquinolones what that do to the potency and dosing profile?
increase systemic circulation and half-life
PRACTICE:
What would happen to ANAEROBE activity if I had a halogen at C8 of Fluoroquinolones? What would happen if I had a methyl group????
HALOGEN at C8- more hydrophilic, MORE anaerobic activity
another ex of a hydrophilic group on C8= OMe
METHYL at C8- more lipophilic, LESS anaerobic activity
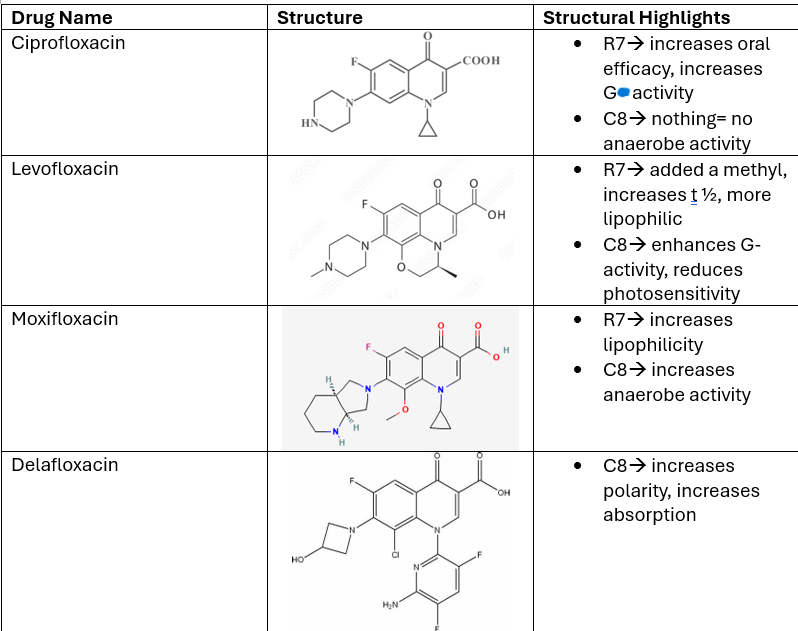
Describe the SAR of CIPROFLOXACIN:
C6
R7
C8
R1
List the groups at each of these positions AND their functions.
C6—> has a FLUORINE= lowers t 1/2, lowers clearance
R7—> PIPERAZINE ring= increases oral efficacy
C8—> METHYL group= more lipophilic, limits anaerobic activity
R1—> cyclopropyl alkyl
What is the impaired renal function dosing of CIPROFLOXACIN? t 1/2?
CrCl 30-50 ml/min —> Q12H
t1/2 ~4 hrs
Describe the SAR of LEVOFLOXACIN:
R7
C8
List the groups at each of these positions AND their functions.
R7—> Nitrogen ring= increases t 1/2, more CNS pen.
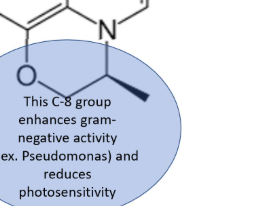
C8—> enhances G- activity, including pseudomonas, reduces photosensitivity
How does the SAR of Ciprofloxacin compare to Levofloxacin?
main difference is in Levofloxacin—> increased half-life, more lipophilic—> CAN PENETRATE CNS
Levofloxacin is less potent than Ciprofloxacin
Describe the SAR of MOXIFLOXACIN:
R7
C8
List the groups at each of these positions AND their functions.
R7—> Nitrogen ring= lipophilic
C8—> OMe group= hydrophilic= more anaerobic activity
What does Delafloxacin have at C8? Results?
has a Cl group—> increases polarity, binding, AND absorption
What is Delafloxacin FDA approved for? (idk how important)
broad spec G+ and G-
acute bacterial skin infections
CAP
BBW of Delafloxacin?
tendon inflammation
peripheral neuropathy
What is a drug interaction with fluoroquinolones? Solution?
polyvalent metal ions (chelation occurs)
give 4 hrs before or 2 hours after
(sing says 2 hrs before, 4 hrs after lowkey i think typo from kearns)
What are 3 mechanisms of RESISTANCE with FLUOROQUINOLONES? Which is specific to Ciprofloxacin?
Qnr gene—> protects DNA gyrase from fluoroquinolones
Bacteria have a variant aminoglycoside acetyltransferase that can modify CIPRO
efflux pumps
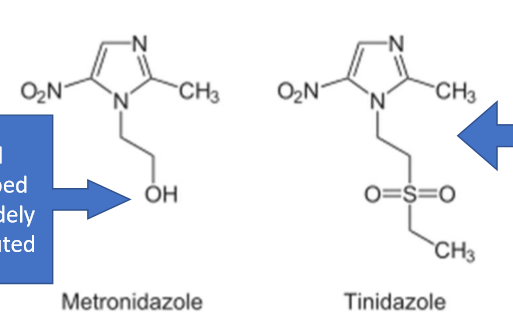
List 2 nitroimidazoles. What type of inhibitors are they?
Drugs: Metronidazole, Tinidazole
DNA synthesis inhibitors
Difference between metronidazole and tinidazole structurally? t 1/2?
tinidazole—> has a SULFUR= gives it a LONGER t 1/2
Nitroimidazoles are effective against what kinds of organisms?
protozoa
bacteria
For nitroimidazoles like Metronidazole and Tinidazole, there is a nitro group at position ___ of the imidazole ring.
5
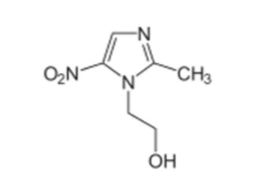
How does metronidazole inhibit bacterial DNA synthesis/MOA?
metronidazole is a PRODRUG—> undergoes a NON-enzymatic reaction, FERREDOXIN reduced—> FERREDOXIN oxidized
this reduction results in toxic metabolites
metabolites incorporated into bacterial DNA
Bacterial DNA is fragmented
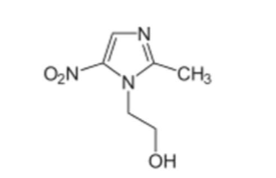
When using metronidazole, the reduction of ferredoxin only occurs in WHAT CELLS?
ANAEROBIC BACTERIA!!!!!!!!
chloroplasts
What would happen if metronidazole got into the soil?
How would you counsel a patient on disposing metronidazole?
in soil—> would kill good bacteria in soil
counsel—> DO NOT put down the toilet
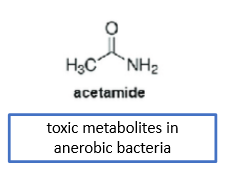
What’s the difference between the active and toxic metabolite of metronidazole?
active—> yes can have an effect on tissues, but NOT therapeutically effective
name: 2-hydroxymetronidazole
hydroxylation, CYP2D6
toxic—> therapeutically effective
name: acetamide
non-enzymatic reduction
Sulfonamides inhibit the pathway bacteria use to synthesize ______________.
folic acid
WHAT are the targets of Sulfonamide AND trimethoprim?
Which enzyme is only in bacteria?
sulfonamide—> Dihydropteroate synthase (DHPS)
ONLY in bacteria
trimethoprim—> Dihydrofolate reductase (DHFR)
in prokaryotes/eukaryotes
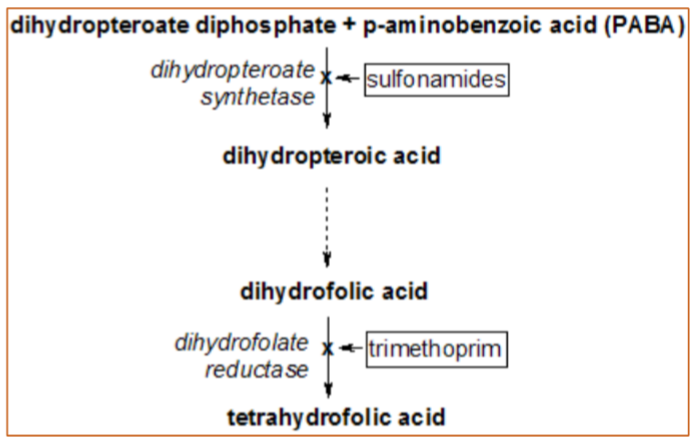
What’s 1 reason why resistance to trimethoprim occurs if the target is DHFR?
nucleotide biosynthesis—> bacteria can utilize the salvage pathway to synthesize ADP, GDP, CDP
What do sulfonamides do to INR? Why?
INCREASES in INR—> bc sulfonamides inhibit metabolism of CYP2C8, CYP2C9
How do anticancer agents targeting the THF pathway differ from sulfonamides?
Anticancer agents targeting the THF pathway are designed to inhibit human enzymes involved in folate metabolism, affecting cancer cell proliferation.
inhibit TS
Sulfonamides specifically target bacterial enzymes, making them selective antibacterial agents.
inhibit DHPS
Pharmacophore of sulfonamides? _______ connected to ________
sulfonyl/amido group connected to amine group
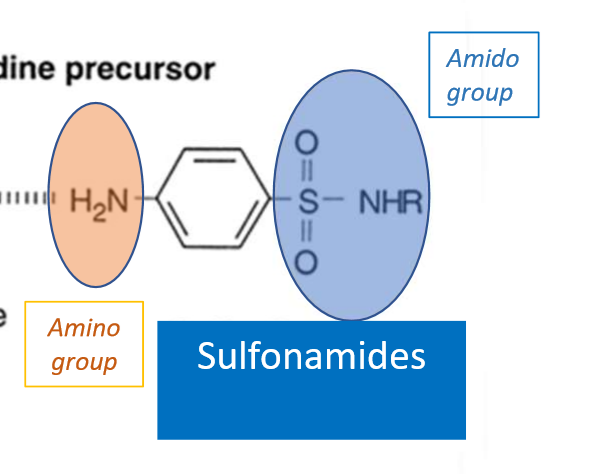
What does the aromatic SO2 group do on sulfonamides?
strongly electron withdrawing—> makes adjacent N partially positive—> makes the molecule electropositive—> makes the molecule acidic
sulfonamides are soluble at ALKALINE pH
What are the therapeutic applications of sulfonamides and trimethoprim?
BROAD SPECTRUM
UTIs, shigellosis, otitis media, traveller’s diarrhea, MRSA, Legionella, bronchitis
Sulfonamides can lower folic acid levels. What are the risks with this?
thrombocytopenia/megaloblastic anemia
hyperkalemia—> due to antagonism at the distal tubule
congenital defects—> can be passed through breastfeeding, can decrease in folic acid metabolism in the fetus
C/I of sulfonamides:
sulfa allergy
renal impairment
anemia
Warnings of sulfonamides:
blood dycrasias
skin rxns
G6PD deficiency
(no G6PD enzyme= can’t convert drug)
Rifamycins:
a. DNA synthesis
b. folic acid synthesis
c. mRNA synthesis
d. protein synthesis
c
Overall MOA of Rifamycins is to inhibit what enzyme?
RNA polymerase-—> specifically DNA-dependent RNA polymerase (DDRP)
Rifamycin antibiotics are not effective for what kind of infections? Why?
systemic infections—> aliphatic chains form a bridge between 2 aromatic moieties
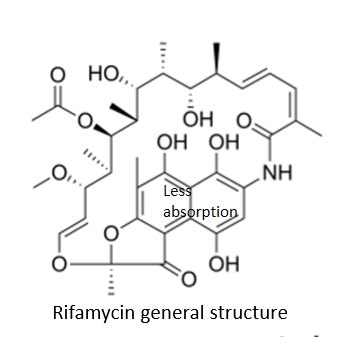
Recognize the general structure of Rifamycin:
is the general structure very hydrophilic or lipophilic?
very hydrophilic
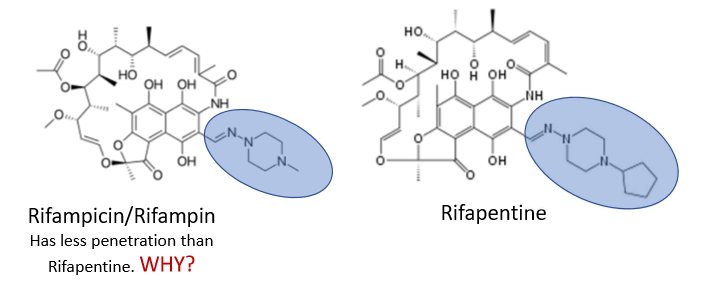
Rifampicin and Rifampin have less penetration than Rifapentine. Why?
rifampicin/rifampin has less alkyl/carbon groups
less lipophilicity
What is special about the binding domain of DDRP?
is a ZINC binding domain (ZBD) and is aromatic
What is the SPECIFIC MOA of Rifamycin?
the naphthene ring on rifamycins form pi-pi bonds with the ZBD to prevent transcription
What happens in state 1 and 2 of transcription to ZBD?
How do rifamycins effect these states?
in state 1—> the ZBD is weakly bound to the promotor region (no transcription)
in state 2—> the ZBD changes its configuration in response to a nearby promoter region (transcription occurs)
this is where rifamycins step in, bind to the ZBD and stop this from happening
Rifamycin should be taken with or without food? Why?
TAKE WITHOUT FOOD/ ON EMPTY STOMACH
Why?
delocalized electrons in the intestine affect absorption kinetics
increased food in the gut can lead to hydrophobic-hydrophobic bonding= decreased absorption
Mechanism of resistance with Rifamycins:
rpoB gene—> bacteria changes its RNA polymerase
results: rifamycins can’t bind to RNA polymerase and inhibit DDRP