AP Biology - Unit 3
🖱 = “click on me!”
Energy ⚡️🖱
Overview 🖱
Energy - the ability to cause change
- types: kinetic, potential; thermal, chemical, light, etc.
Thermodynamics - the study of energy transformations
- system types: isolated (no environment interaction), open (environment interaction)
- laws: 1st - energy can only be transferred/transformed, not created/destroyed; 2nd - energy transfer/transformation increases entropy (energy dispersal within universe)
Processes - spontaneous (occur without energy input), nonspontaneous (require energy input)
(Gibbs) Free Energy - energy available to do work (∆G = G(final) - G(initial))
- -∆G = spontaneous, +∆G = nonspontaneous
Reactions - %%endergonic%% (net gain of energy), %%exergonic%% (net release of energy)
%%Metabolism%% - the energy use of an organism
- main energy supplier - %%Adenosine Triphosphate%% (through phosphorylation (releasing a phosphate group))
%%Catabolic%% - breaking down large molecules into smaller ones
%%Anabolic%% - building up large molecules from smaller ones
Enzymes 🧪🖱
Enzyme Function 🖱
%%Enzymes%% act as catalysts by reducing the activation energy required for a reaction to take place. Enzymes have high %%specificity%% (their shape is specific to a substrate)
%%Activation Energy%% is required to bring a reactant to its transition state
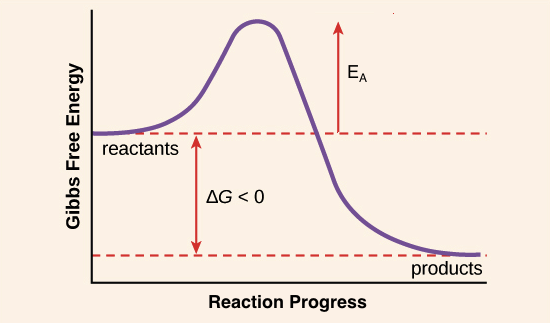
Catalysis - the speeding up of a reaction
Enzyme Parts 🖱
Substrate - the reactant being catalyzed (into the product)
Active Site - where a substrate attaches to an enzyme
Cofactor - molecules that aid an enzymes function
Coenzymes - enzymes that aid an enzymes function
Inhibitors - attach to enzymes (affect functions, usually negative)
- competitive (attach to the active site, prevent substrates from entering)
- noncompetitive (attach to other areas (allosteric sites) and change the shape to prevent substrates from entering)
- selective (beneficial)
Allosteric Regulation - when regulatory molecules affect a protein’s function
Feedback Inhibition - when products are used to prevent catalysis (turning a reaction “off”)
Multi-enzyme Complex - an enzyme “assembly line”
Factors 🖱
Temperature, pH, and other chemical conditions affect the ability of an enzyme to function (can cause denaturation)
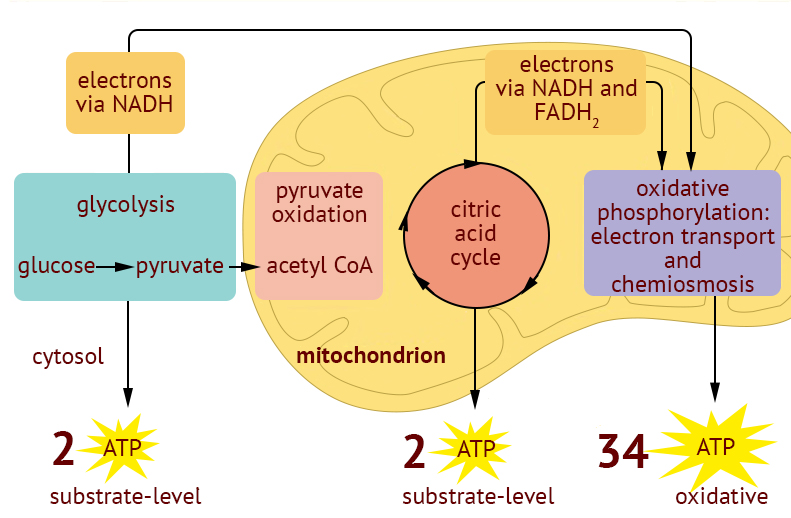
Cellular Respiration 🫁🖱
Overview 🖱
Cellular Respiration Formula: C₆H₁₂O₆ + 6O₂ → 6CO₂ +6H₂O + energy (ATP)
- Aerobic (with oxygen)
Respiration is a redox (oxidation reduction) reaction - one reactant gains electrons (reduction) and another loses electrons (oxidation)
The main electron carriers of the system are NAD+ and FAD
~38 ATP are formed
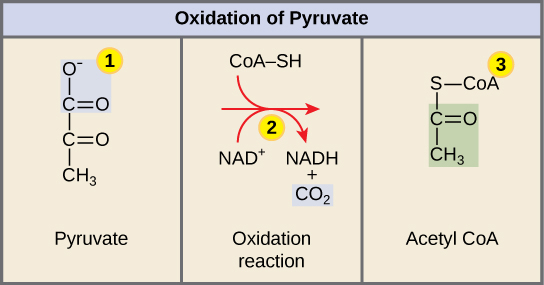
Glycolysis and Pyruvate Oxidation 🖱
Glycolysis Formula: Glucose + 2ATP →(energy investment)→ fructose1,6-bisphosphate + 2ADP + 2(P)ᵢ + 2NAD+ + 4e- + 4H+ →(energy payoff)→ 2Pyruvate + 4H₂O + 4ATP + 2NADH + 2H+
Glycolysis Location: Cytosol
Pyruvate Oxidation (Link Reaction) Formula: 2Pyruvate + NAD+ + H+ + 2CoenzymeA → 2Acetyl-CoA + NADH
Pyruvate Oxidation Location: Mitochondria
Energy Investment (gets glucose to a transition state) - uses 2 ATP
Energy Payoff (turns the glucose into Pyruvate) - gains 4 ATP and 2NADH

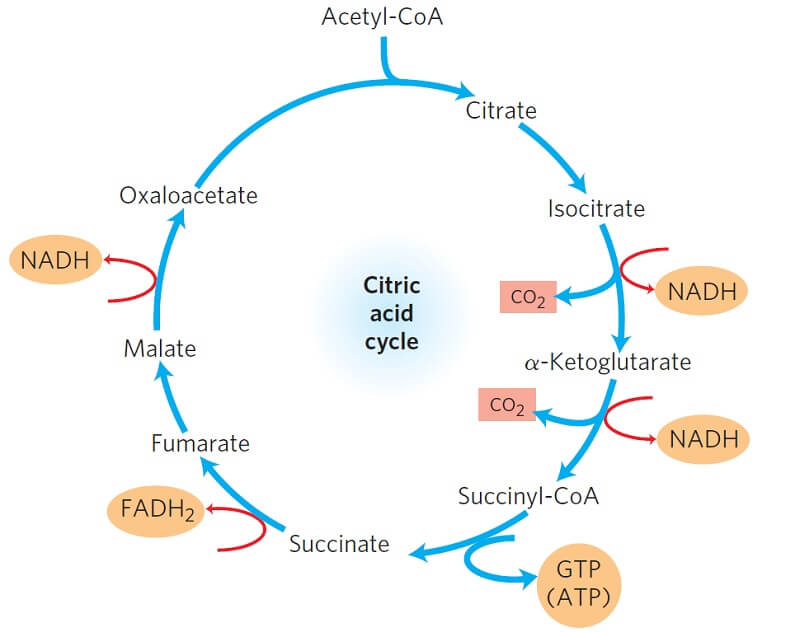
Citric Acid / Krebs Cycle 🖱
Citric Acid Cycle Formula: 2Acetyl-CoA + 6NAD+ + 2FAD + 2ADP + 2(P)ᵢ + 4H₂O → 2CoA-SH + 6NADH + 2FADH₂ + 2ATP + 3H+ + 4CO₂
Citric Acid Cycle Location: Matrix
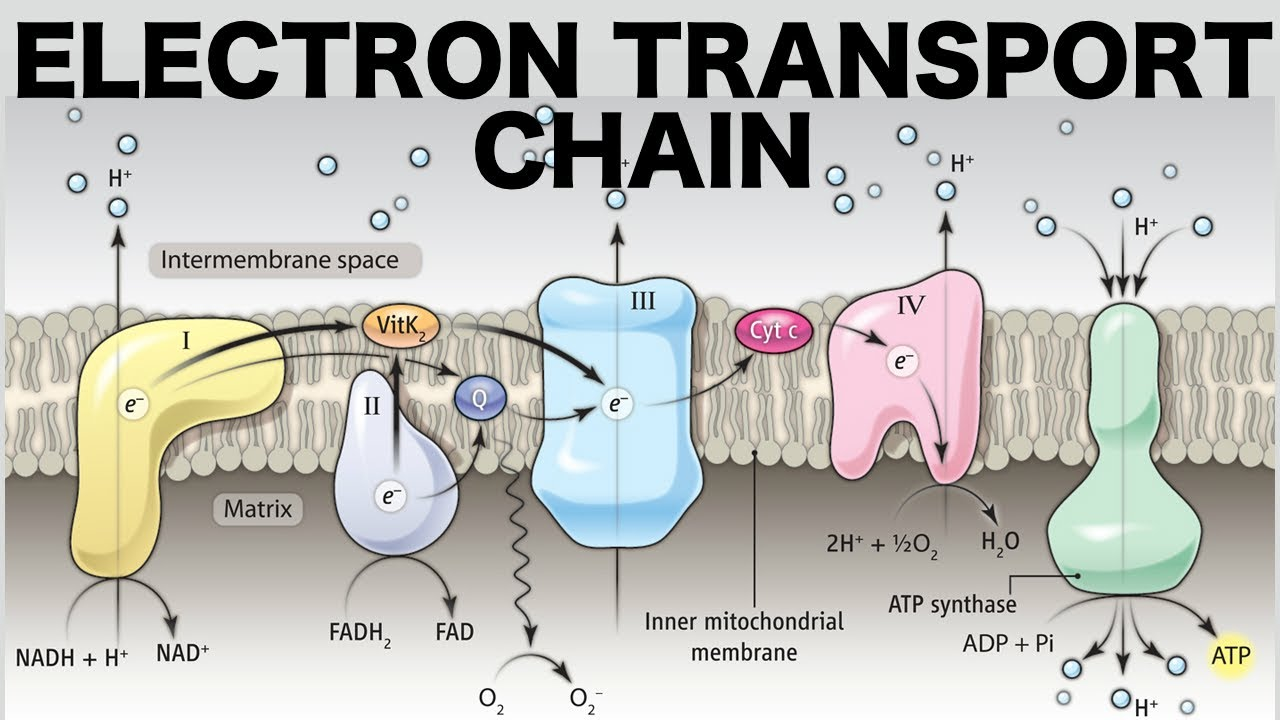
Oxidative Phosphorylation (Electron Transport Chain and Chemiosmosis) 🖱
Electron Transport Chain Function: electrons are added to the chain and create a pH/H+/proton gradient in the intermembrane space
Chemiosmosis Function: H+ ions pass through ATP synthase as a proton-motive force to complete (ADP + (P)ᵢ → ATP)
Oxidative Phosphorylation Location: Inner Membrane (& Matrix/Intermembrane Space)
Electron Transport Chain Process: NADH drops off two electrons to protein complex I, and FADH₂ drops off two electrons to protein complex II; as the electrons travel between proteins, they release H+ ions into the intermembrane space; the final electron acceptor is (1/2)O₂ which combines with 2H+ to form H₂O
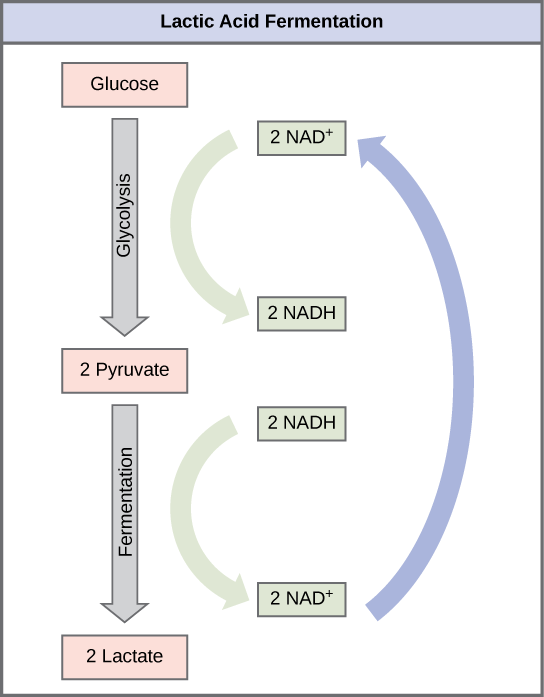
Anaerobic Respiration and Fermentation 🖱
Anaerobic Respiration - when the respiration process lacks oxygen (electron transport chain either uses a non-oxygen electron acceptor (bacteria), or carries out fermentation)
Fermentation - without oxygen, sometimes mitochondria skip the steps beyond glycolysis, and form less ATP through just glycolysis (to do this, they must turn the resulting Pyruvate into an electron acceptor to recycle NAD+)
Alcohol Fermentation: Pyruvate is converted into Acetaldehyde which accepts electrons from NADH to form Ethanol
Lactic-Acid Fermentation: Pyruvate accepts electrons from NADH to form Lactate
Obligate Anaerobes - must use anaerobic respiration
Facultative Anaerobes - can use anaerobic respiration
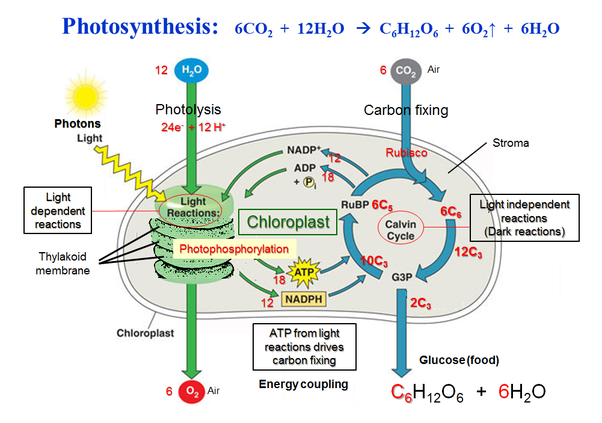
Photosynthesis 🌱🖱
Overview 🖱
Photosynthesis Formula: 6CO₂ + 12H₂O + light → C₆H₁₂O₆ + 6O₂ + 6H₂O
- mainly in Mesophyll cells (high chloroplast count)
Plants get CO₂ from stomata in their leaves
The main electron carrier of the system is NADP+
The energy resulting from the process is stored in the chemical bonds of glucose (used in cellular respiration)
Light-dependent Reactions 🖱
Light 🖱
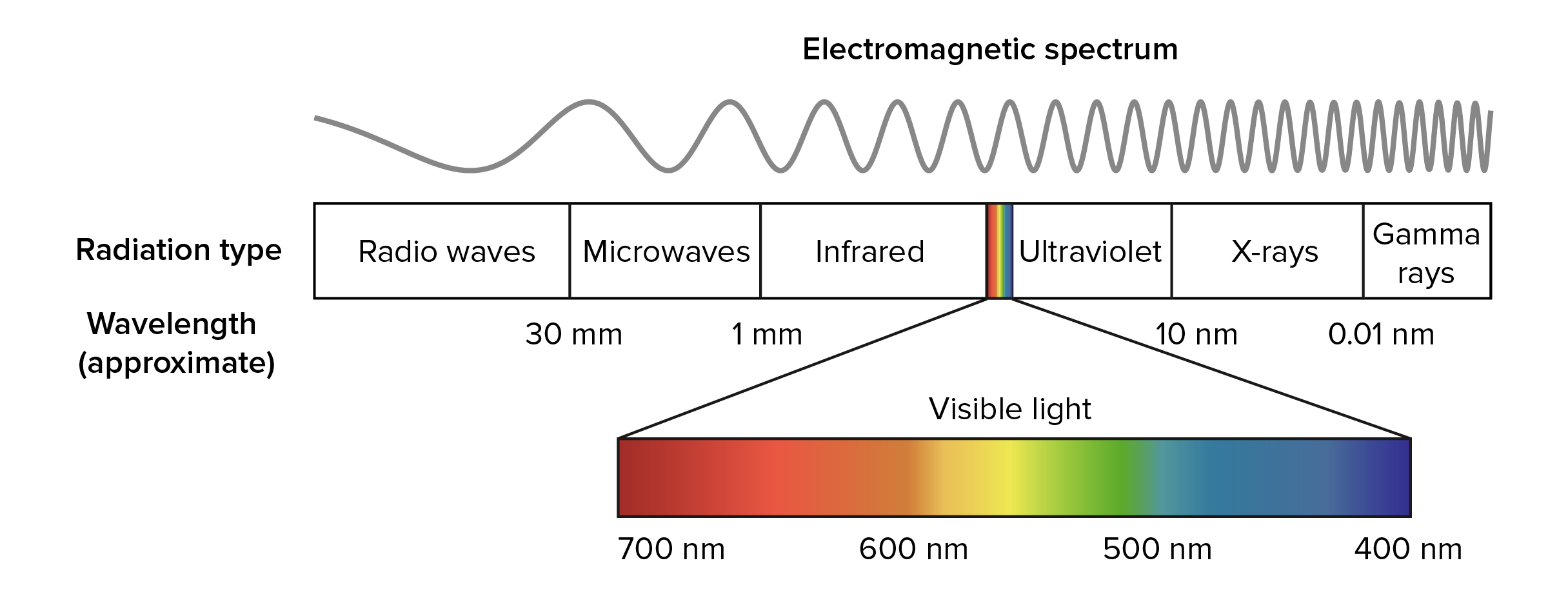
Light energy exists in the form of photons which act as particles that can excite electrons
Pigment reflect the color that they appear as, and absorb all other wavelengths of light