Osmolarity
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Concentration
number of solutes / volume
Solutes
anything that can dissolve in a given volume of liquid
What causes diffusion of solutes?
Brownian motion: solutes are antisocial and need room for each other —> so solutes will distribute evenly for equilibrium (both sides are equal concentration)
If you have more concentration, will receptor bind more or less to solute?
More ; more concentrated will activate receptor more because there is more chance to bind to receptor
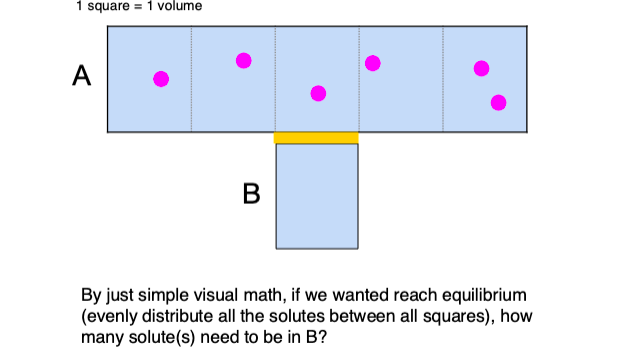
1 solute
A —> 5 solutes / 5 volume = 1 solute/volume
B —> 1 solute / 1 volume = 1 solute/volume
How do nonpermebale solutes adjust to reach equilibrium?
Water can still diffuse across in order to reach equals concentration (osmosis)
Osmosis
water moves from low solute to high NP solute concentration
water helps equilibrate solute concentrations when they are nonpenetrating
Types of solutes
Penetrating: urea
Non penetrating: ions (Na+, K+)
Partially penetrating: glucose (can penetrate the membrane, but once inside gets trapped because of phosphorylation)
Osmoles
takes into consideration the dissolution of the solute in solution
Osmolarity
compares any 2 solutions and describes a solution concentration
Mechanisms of equilibrium:: diffusion, osmosis
does not matter nature of solute
Units: OsM
Hyperosmotic
Hypoosmotic
Isoosmotic
Hyperosmotic: a solution has more osmolarity than another solution
Hypoosmotic: a solution has lower osmolarity than another solution
Isoosmotic: two solutions have same osmolarity
Tonicity
Uses: Describes a solution compared to a cell only
Describes how that solution will affect behavior of cell - whether a solution will cause water to move out of cell or not (shrink, swell, etc)
Mechanism of equilibrium: osmosis
Nature of solute matter: Yes - only NP solute (as NP allows water to equilibrate to create equilibrium)
Units: None
Hypertonic
Isotonic
Hypotonic
Hypertonic solution: water moves out of cell → causes cell to shrink
Isotonic: no net movement of water → normal cells
Hypotonic: water moves into the cell → cells swell and eventually burst
If there is no NP solute in solution
cell will burst if its hypotonic
Total body water
33% is extracellular fluid: plasma (8%) and interstitial fluid (25%)
67% is intracellular fluid
Markers
indicate volumes of body compartments
D20: measure TBW
Insulin: measure ECF
Evan’s blue: measure plasma
Physiology reference man
155 lb or 70 kg
TBW: 60% of 70 L = 42 L
Plasma Osmolarity: 300 mOsm
Volumes of distribution: 14 ECF and 28 ICF
Dehydration
Loss of hypoosmotic solution = lose more water than solute
concentration of ECF has increased, water move from ICF to ECF to equilibrate
Hemorrhage
extreme blood loss: losing same proportional amount of water and solid
Lose isosmotic solution: no change in concentration
Priorities to treat dehydration
Regain volume loss for circulatory system (ex blood pressure) in ECF
Rehydrate cells to regain proper cell function
Then lower osmolarity
How to assess initial cell response
Calculate concentration
Look at osmolarity If hyperosmotic: cell will shrink
If hypoosmotic: cell will swell
If isosmotic cell will not change
Now calculate the concentration of non penetrating solutes outside compared to inside
Hypertonic = NP outside greater NP inside
Cell shrinks
Hypotonic = NP outside less than NP inside
Cell swells
Cell burst/hemolyze if NP outside = 0
Isotonic = NP outside = NP inside
Cell no change
IV therapy
replenish fluid loss
Normal Saline
0.9% saline
Isomotic
Isotonic
Normal saline sits at 300 mOsm which reflects osmolarity of the body
Best for hemorrhage patient
D5 normal saline
5% dextrose in 0.9% saline
Hyperosmotic
Isotonic
D5W
5% dextrose in water
Isomotic
Hypotonic
Half normal saline
0.45% saline
Hyposmotic
Hypotonic
D5 half normal saline
5% dextrose in 0.45% saline
Hyperosmotic
Hypotonic
What solutions is isotonic?
Normal saline
D5 normal saline
What solutions is hypotonic
D5W
½ normal saline
D5 ½ normal saline
Treat dehydration
Hyposmotic fluid loss from ECF and ICF
Hyposmotic, hypotonic solution with NP solutes
Hydrates cells but keeps some fluid in plasma
Treat hemorrhage
Isomotic fluid loss from ECF only
Isotonic solution