5.2.3 Redox and electrode potentials
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
14 Terms
oxidising agent
accepts electrons and gets reduced
reducing agent
donates electrons anf gets oxidised
how to construct half equations
check for exceptions
check if the atom undergoing change in oxidation number change is balanced and if not balance
remember to take into account balancing when calculating oxidation number change
for oxidation electrons written on products
reduction electrons on reactants side
how to contrsyt redox equations
check if both half equations have same numebr of electrons ifnot multiply
add 2 half equtions
check charges on both sides
if not balances add h+/OH- to reactants and water to products
check if numebr of atoms are bakances
differences with redox titrations
In many cases, no additional indicator is needed as the reactants themselves undergo colour changes that signal the end point. This is known as a self-indicating titration.
The titration determines the amount of oxidising agent needed to exactly react with a given quantity of reducing agent (or vice versa).
why are transition metals good redox reagent s
cThey can readily change oxidation states by accepting or donating electrons, making them effective oxidising or reducing agents.
These changes in oxidation state are often accompanied by distinct colour changes, which serve as built-in visual indicators.
The colour changes make it easy to identify the end point of the titration without the need for an additional indicator in many cases.
half equations for reduction of permanganate ion
MnO4- + 8H+ + 5e- ➔ Mn2+ + 4H2O
half equation for oxidation of fe2+
Fe2+ ➔ Fe3+ + e-
full equation for Redox titrations with potassium permanganate

Titration procedure for determining MnO4- concentration
Measure a known volume of the reducing agent solution (e.g., Fe2+) using a pipette into a conical flask. Add an excess of dilute sulfuric acid to ensure sufficient H+ ions for MnO4- reduction.
Gradually add MnO4- solution from a burette to the flask, swirling constantly. Stop when the mixture becomes faintly tinted with the purple MnO4- colour, which indicates the end point. Record the volume of MnO4- added.
Repeat the titration until concordant results are obtained (titre volumes that differ by no more than 0.10 cm3).
Calculate the mean volume of MnO4- added from the concordant titres.
half equations for iodine-thiosulfate redox titrations
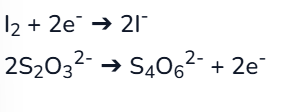
full eqaution for iodine-thiosulfate redox titration

3 main stages of iodine-thiosulfate redox titration
A known volume of the oxidising agent is reacted with an excess of acidified potassium iodide (KI) solution. This oxidises some of the iodide ions (I-) to iodine (I2).
The iodine produced is then titrated with a sodium thiosulfate (Na2S2O3) solution of known concentration. The thiosulfate ions (S2O32-) reduce the iodine back to iodide ions.
The concentration of the original oxidising agent is calculated using the volume and concentration of the sodium thiosulfate solution used in the titration.
how does the colour chnage occur in sodium thiosulfate titration
Here's how the colour change occurs:
During the titration, the dark brown colour of the iodine solution gradually fades as the iodine (I2) is reduced to colourless iodide ions (I-) by the thiosulfate ions (S2O32-) from the sodium thiosulfate solution.
When the majority of the iodine has been reduced and only a small amount remains, a few drops of starch solution are added to the titration mixture. Starch forms a deep blue-black complex with iodine, making it much easier to detect the presence of even trace amounts of iodine in the solution.
As more sodium thiosulfate is added, the blue-black colour persists until all the remaining iodine is reduced to iodide.
At the end point of the titration, the final drop of sodium thiosulfate reduces the last trace of iodine, causing the blue-black colour to disappear suddenly and completely, leaving a colourless solution.
his sharp colour change from blue-black to colourless is easily recognisable and indicates that all the iodine has been consumed, marking the end point of the titration.