UGA Chem1211 Polyatomic Ions
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
phosphate
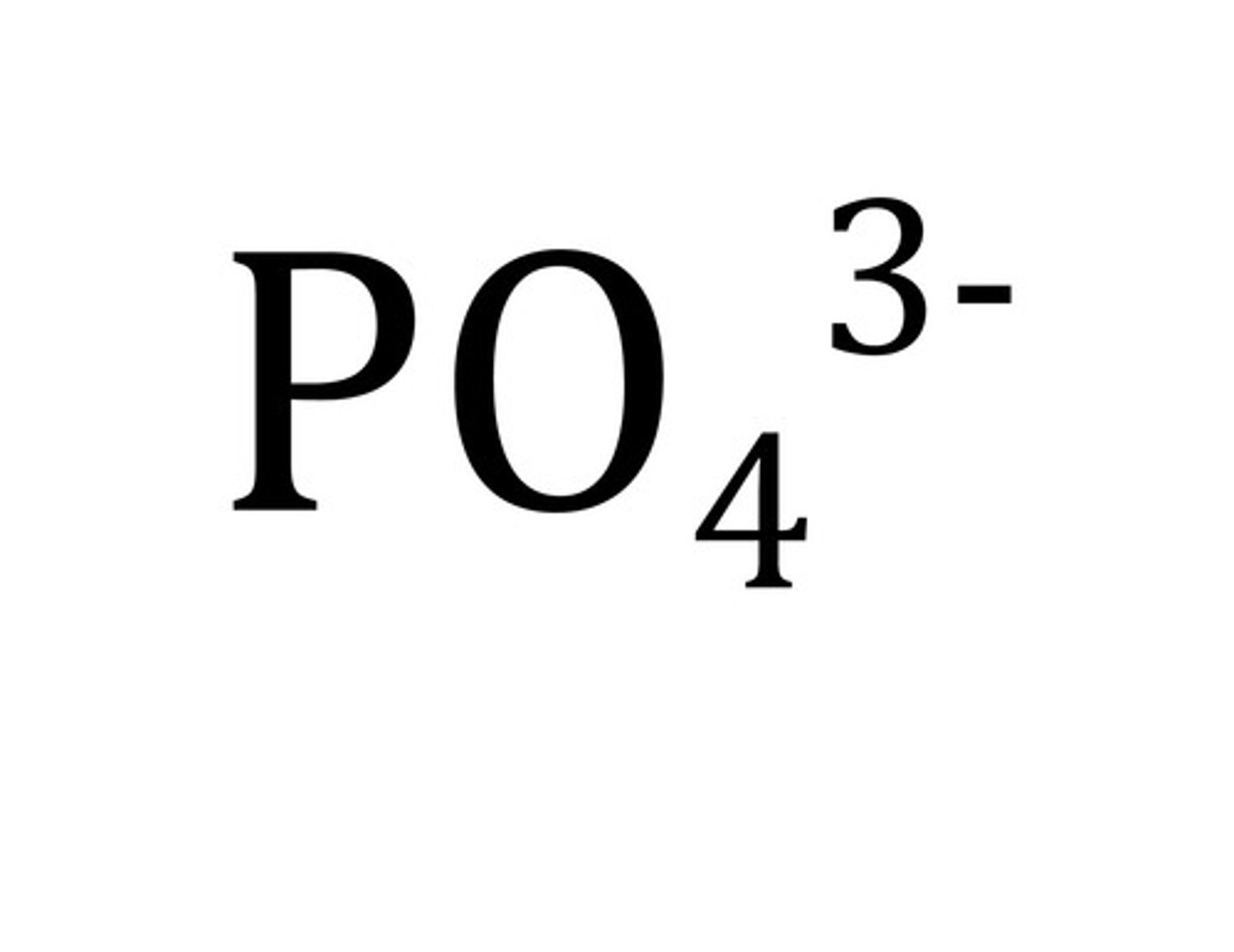
hydroxide
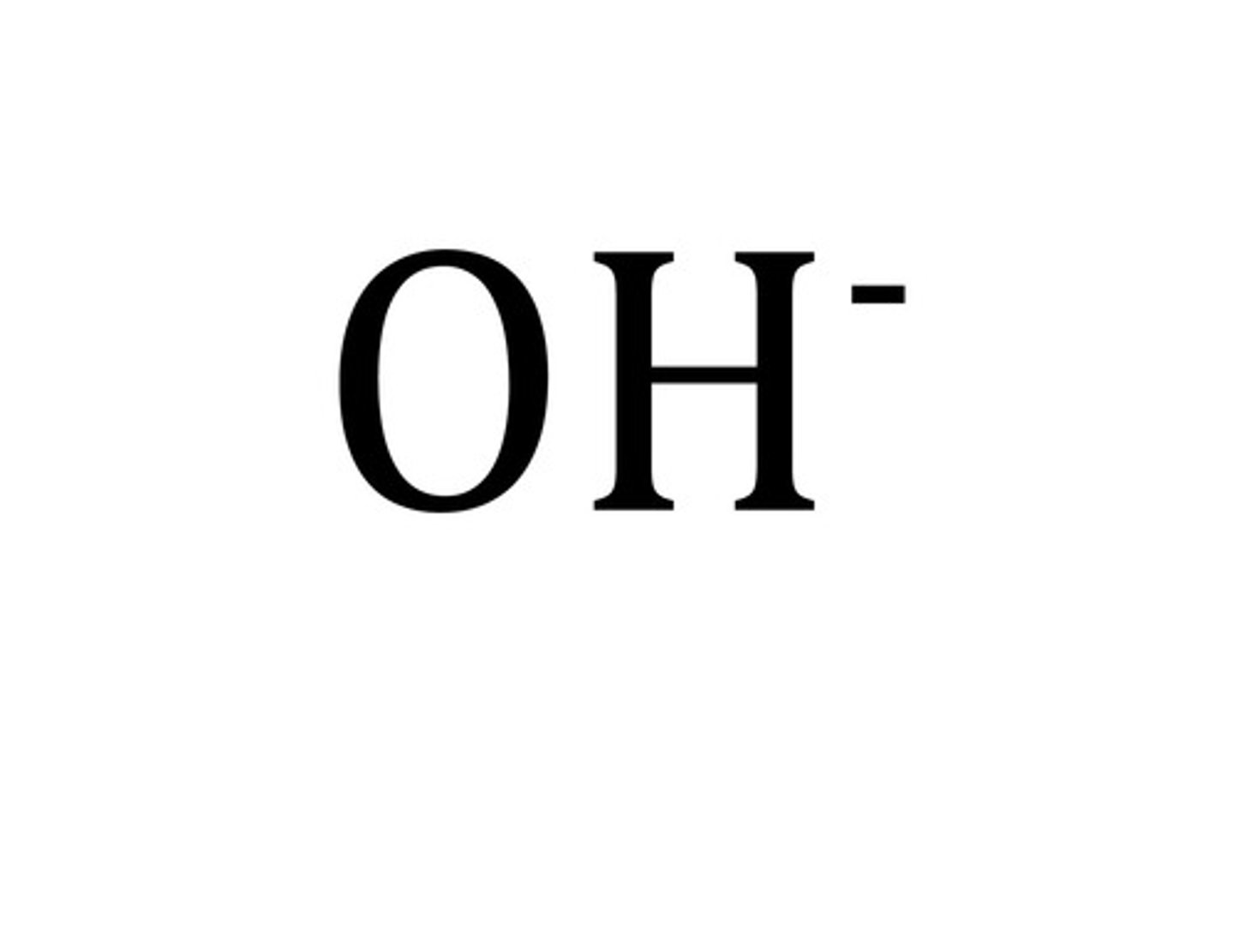
sulfate
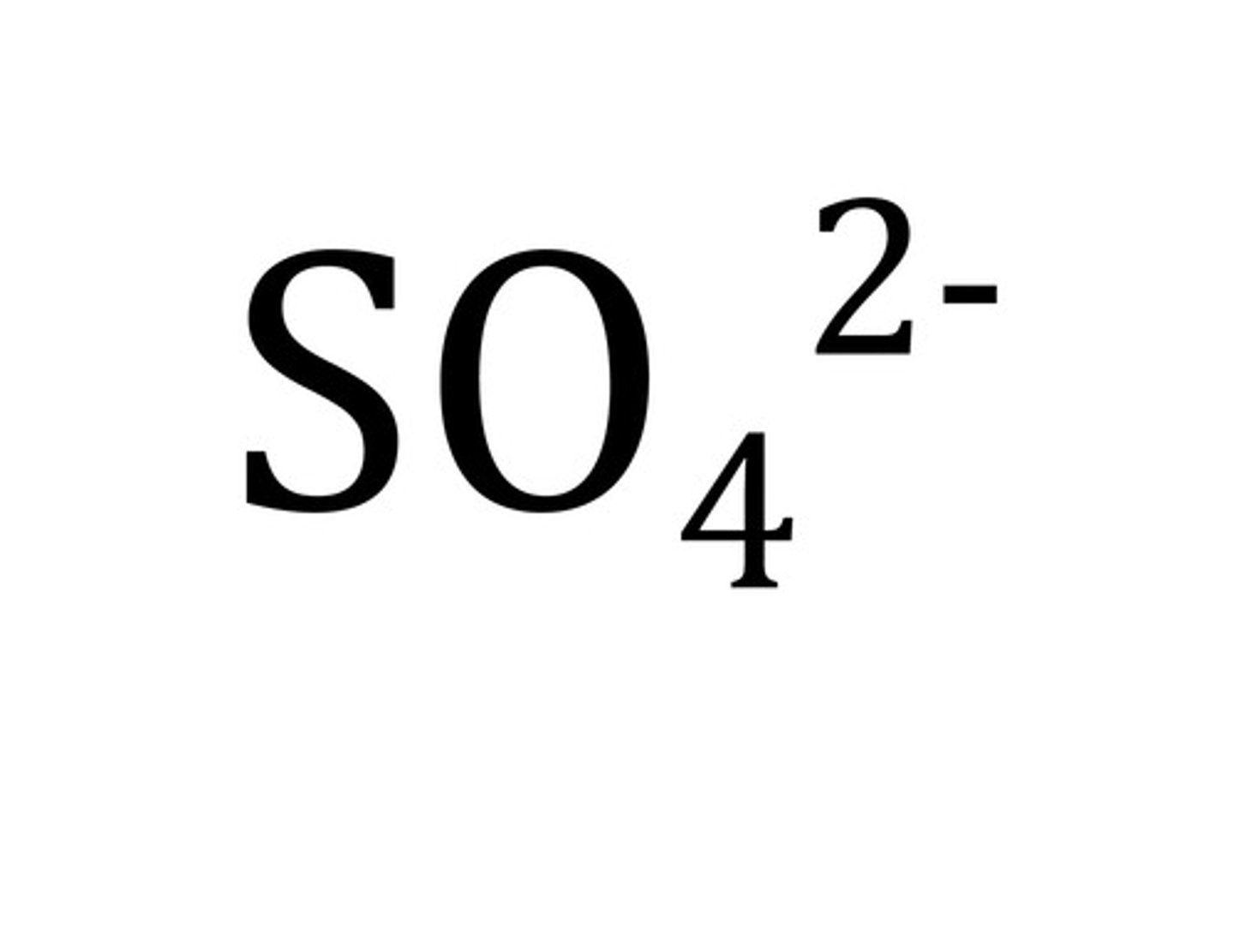
acetate
CH3COO 1+
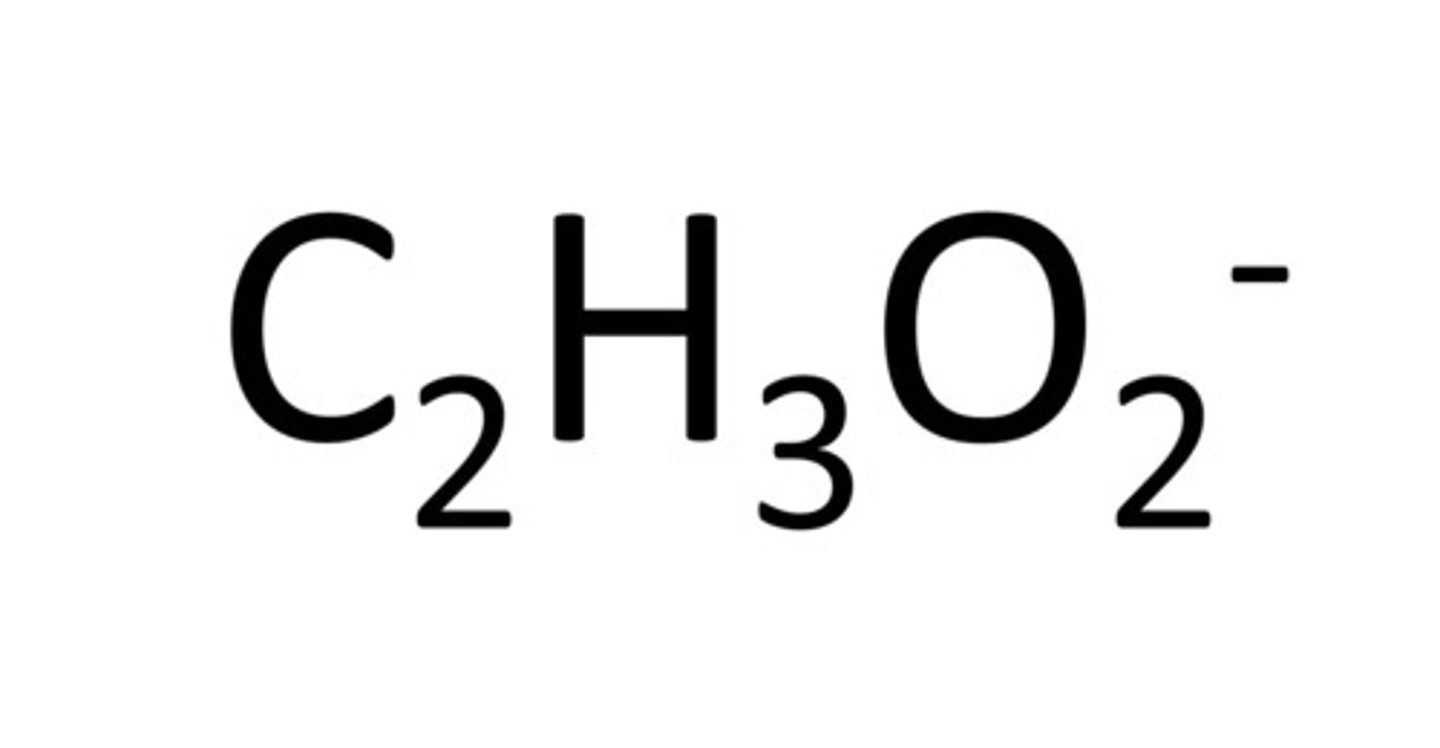
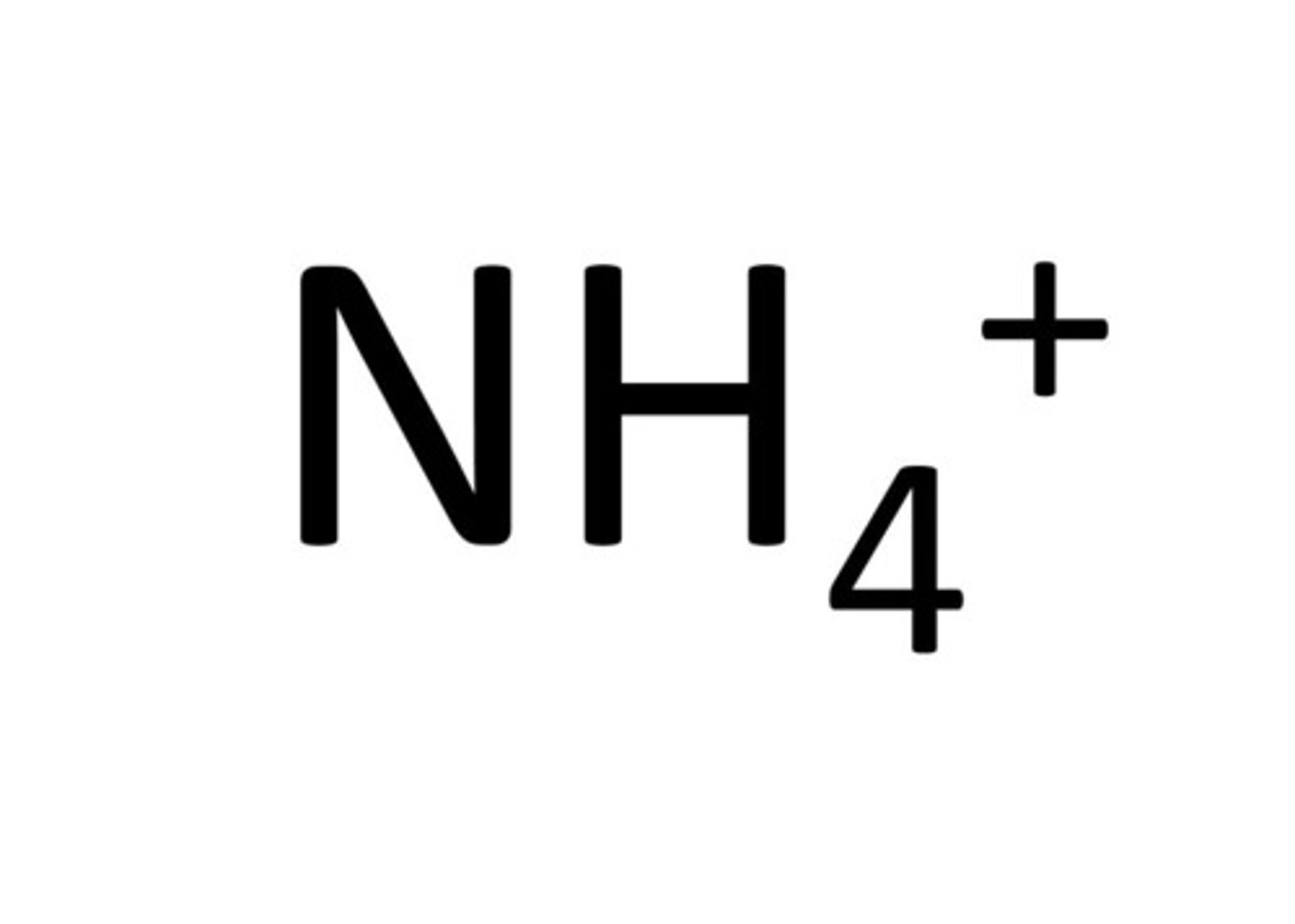
ammonium
chlorate
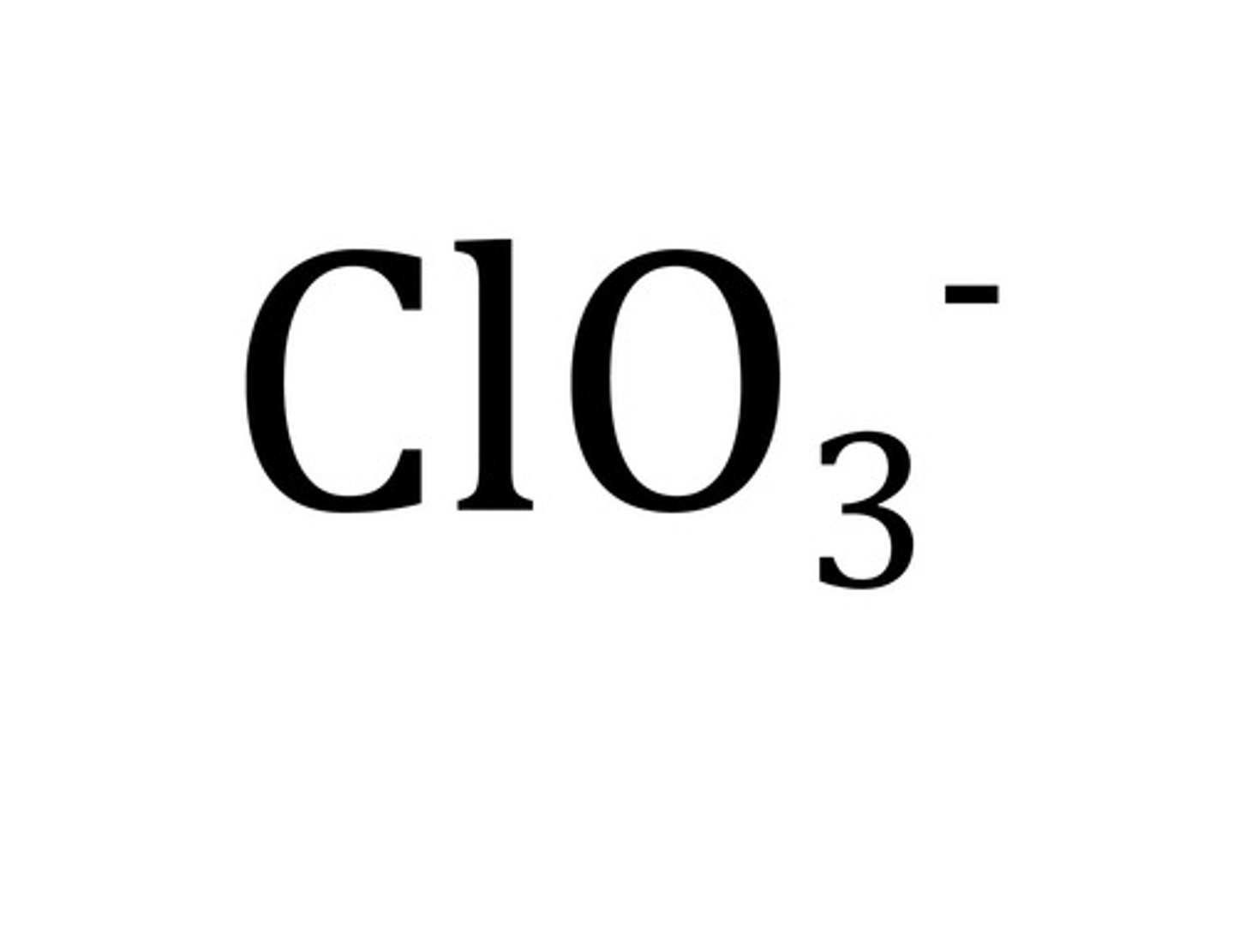
nitrate
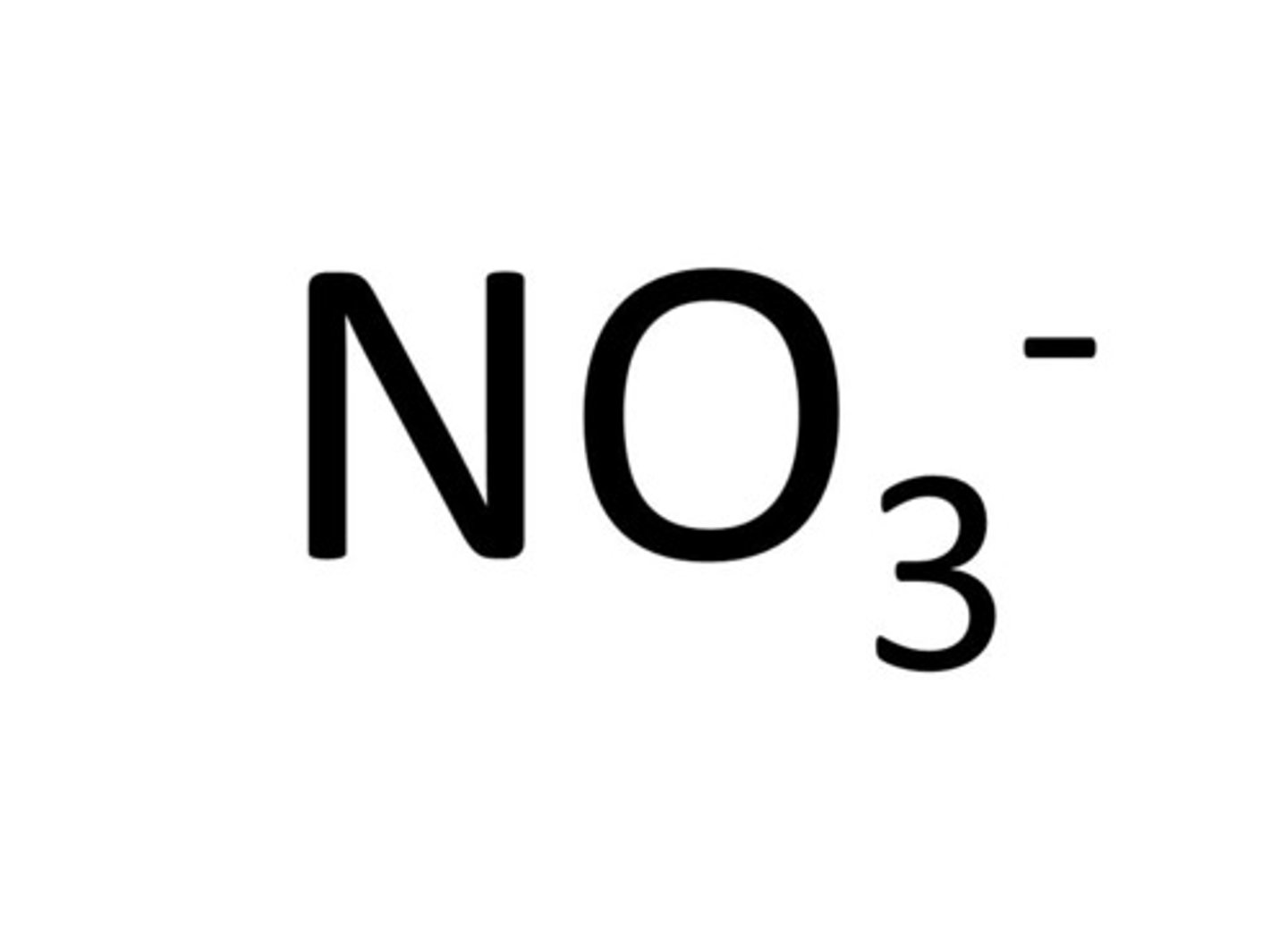
permanganate
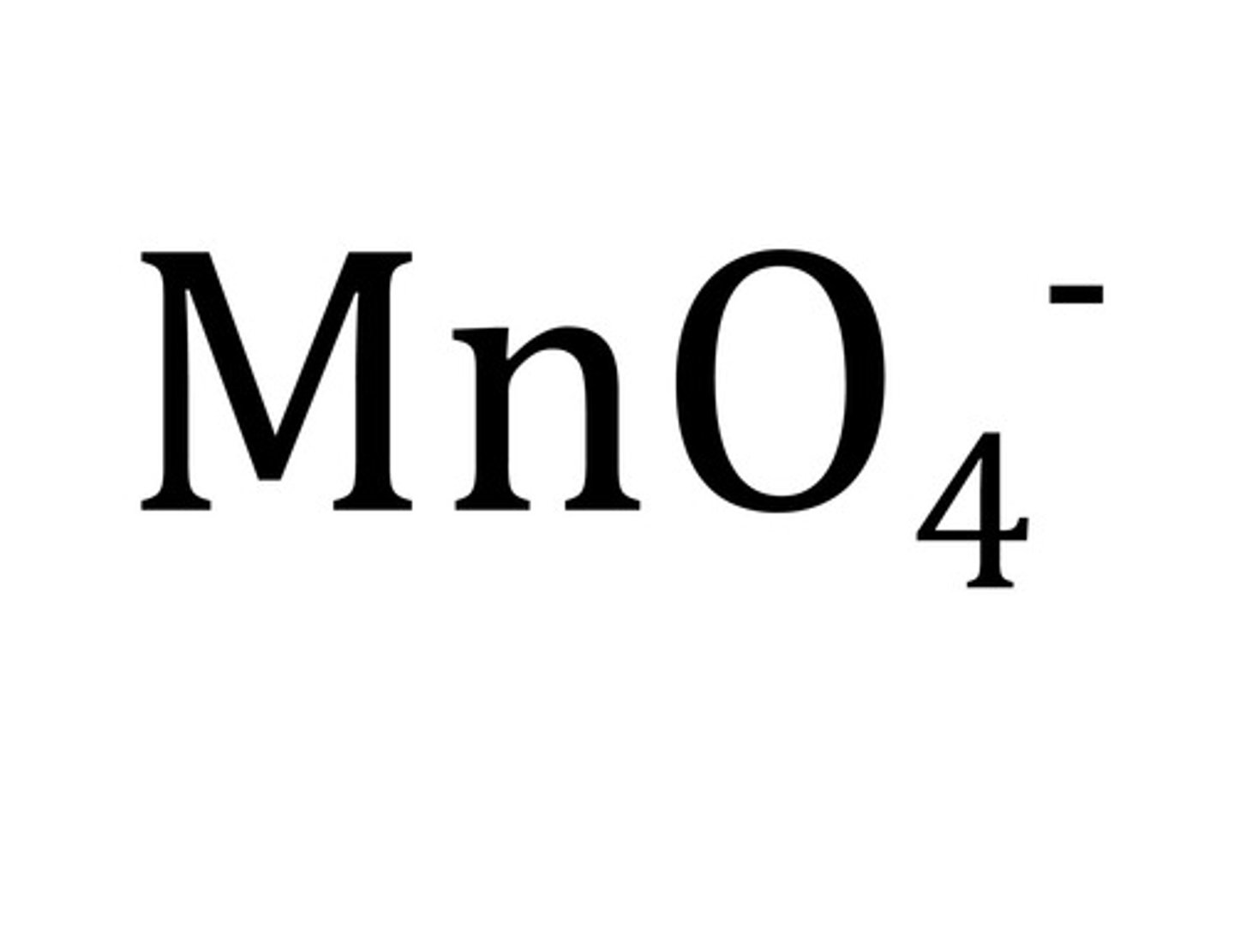
chromate
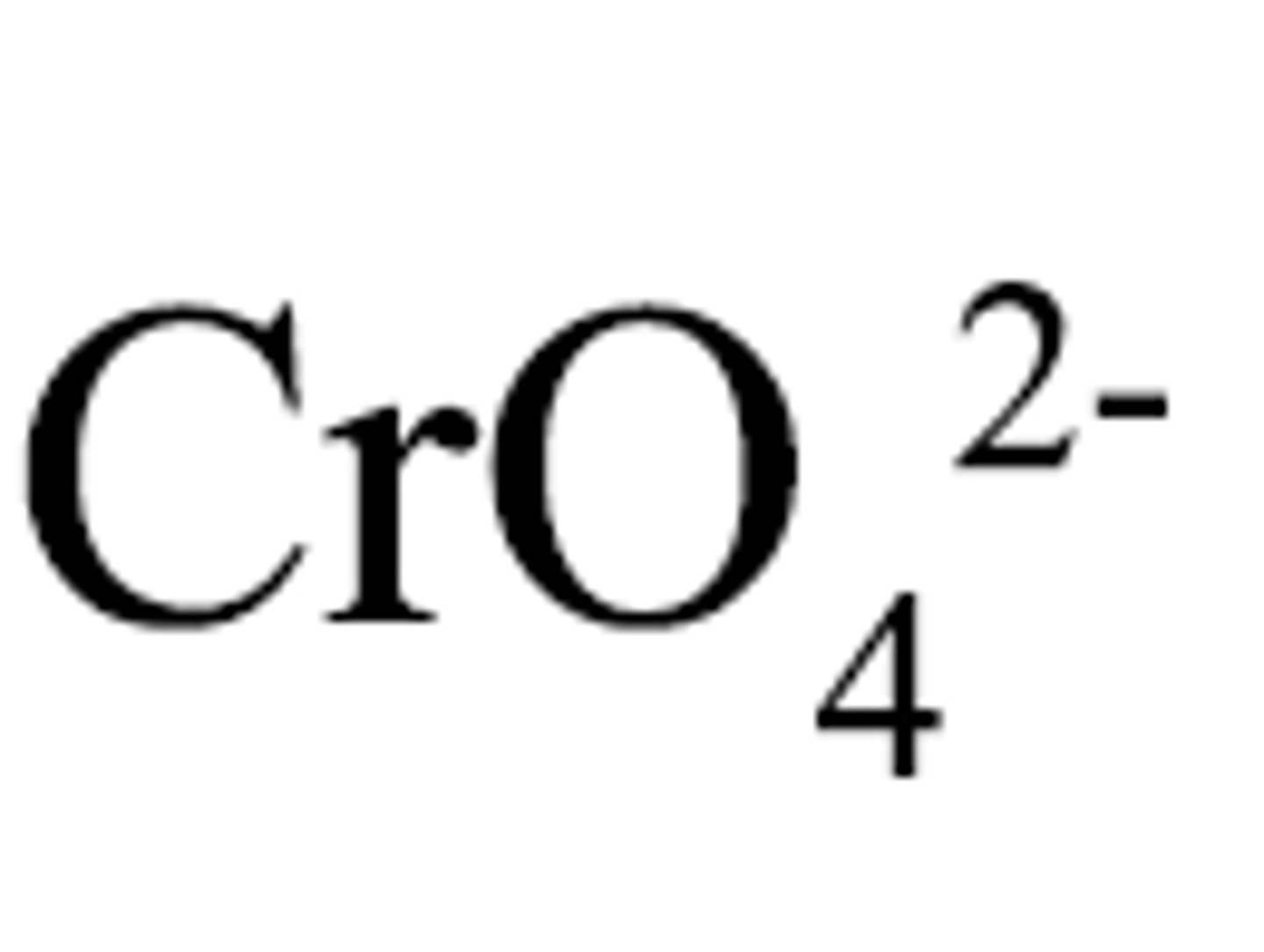
carbonate
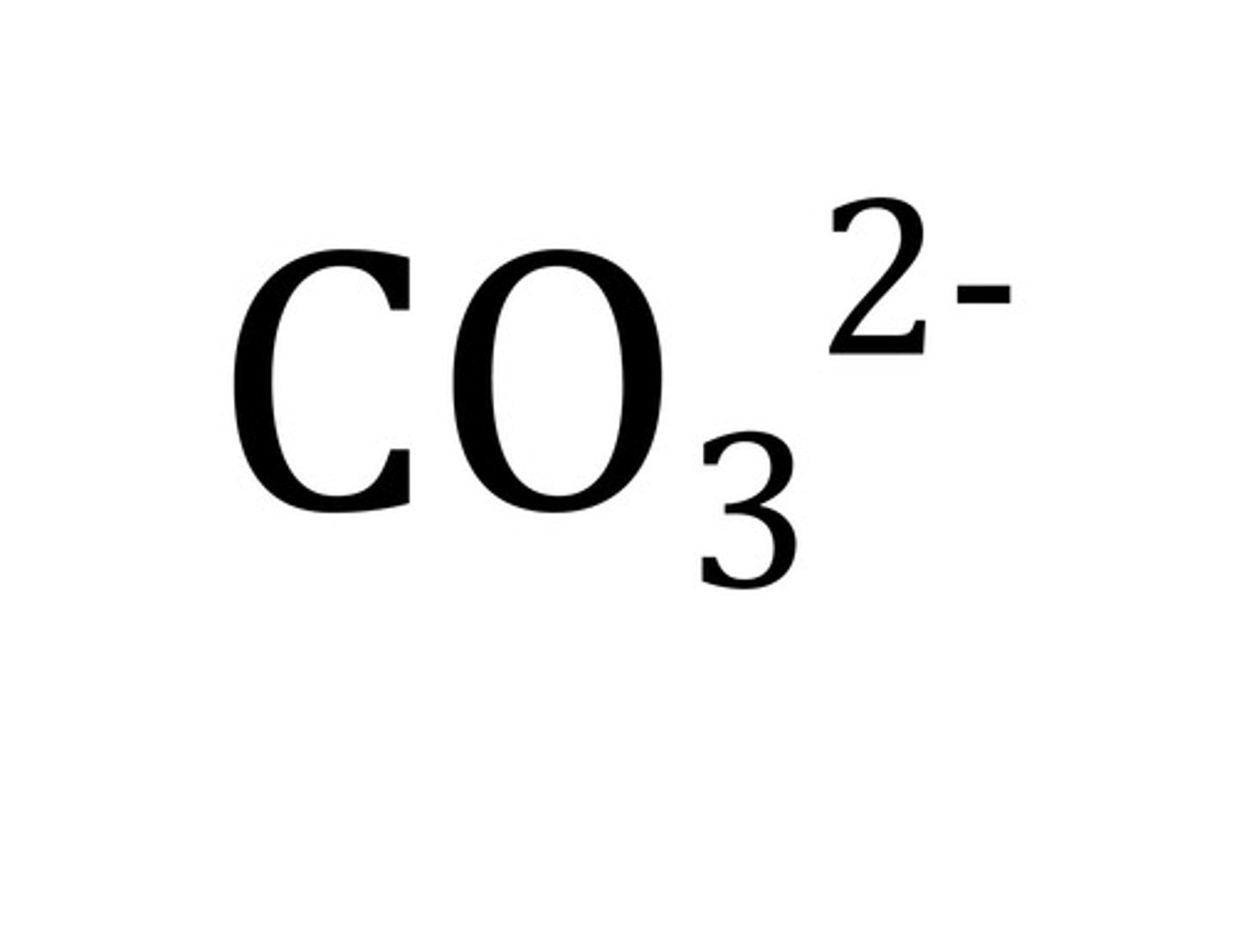
cyanide

dichromate
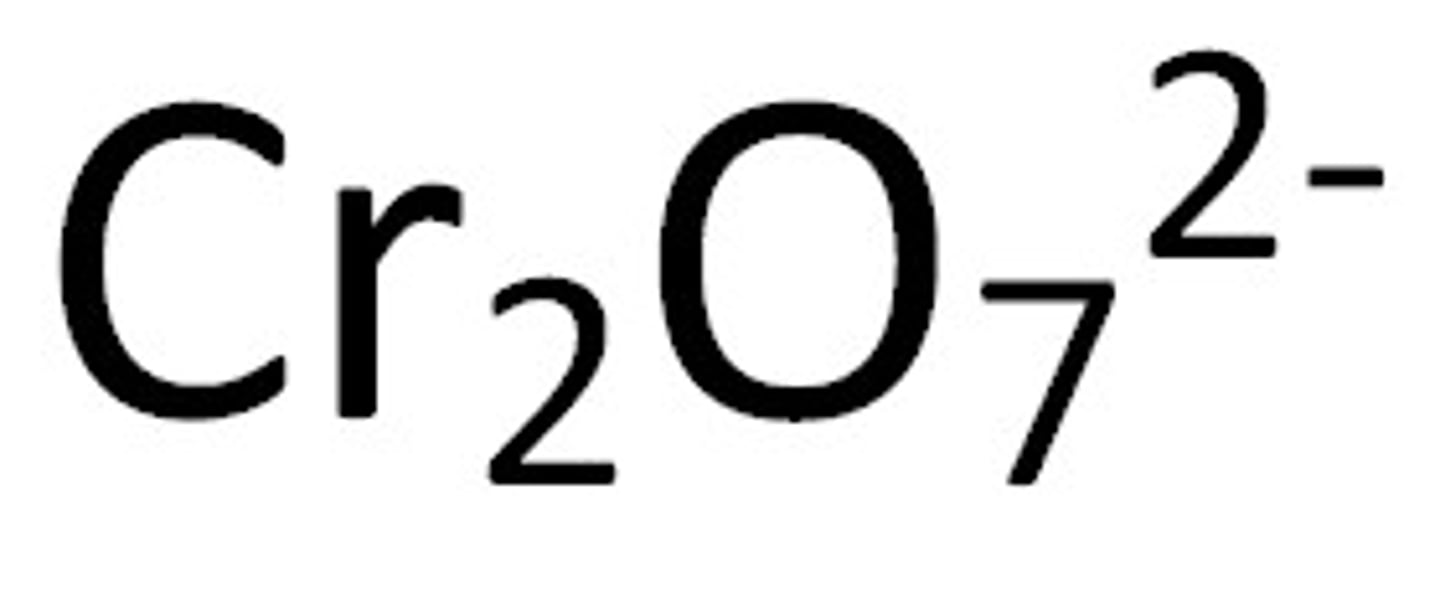
carbonate
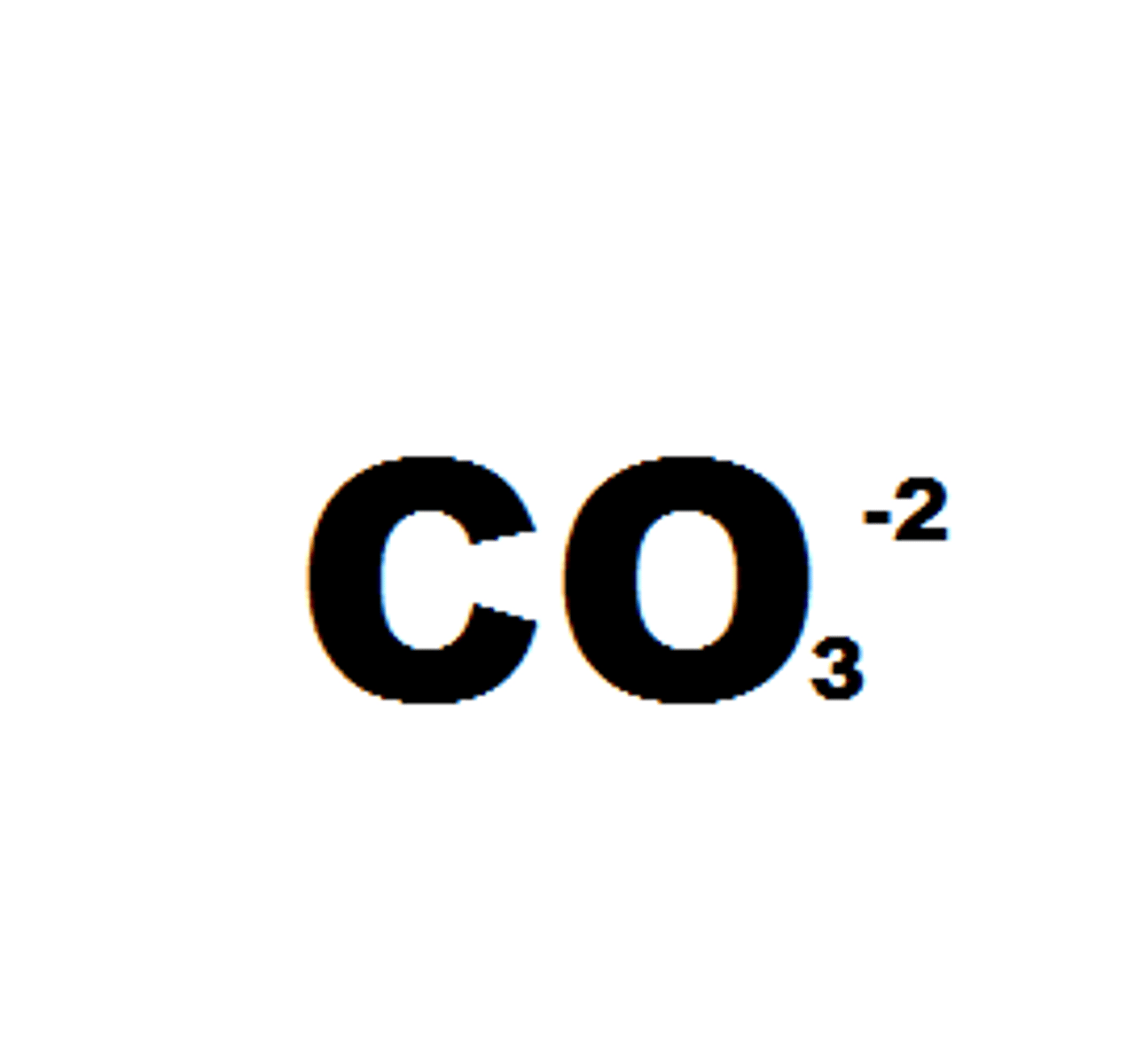
nitrite
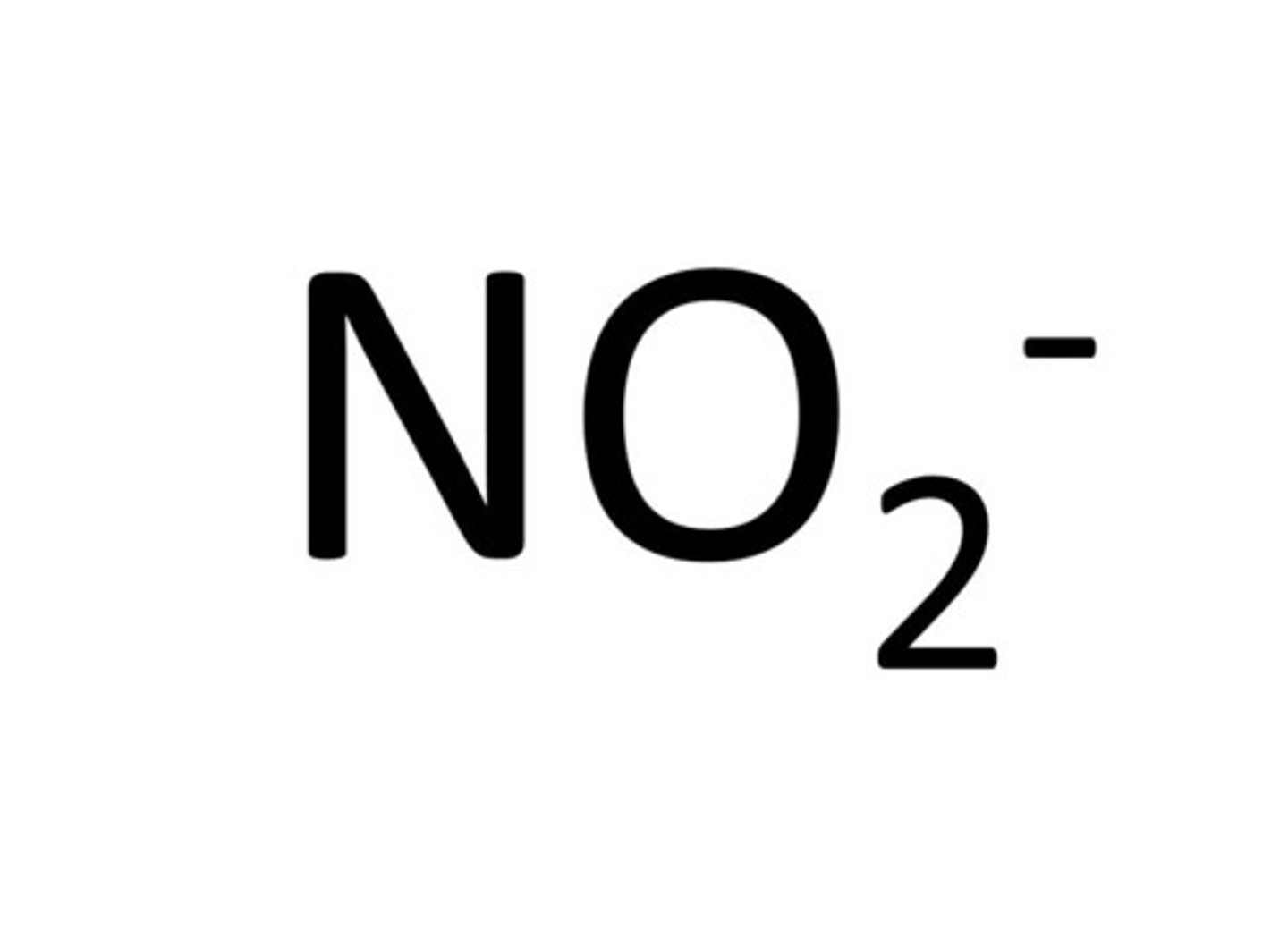
sulfite
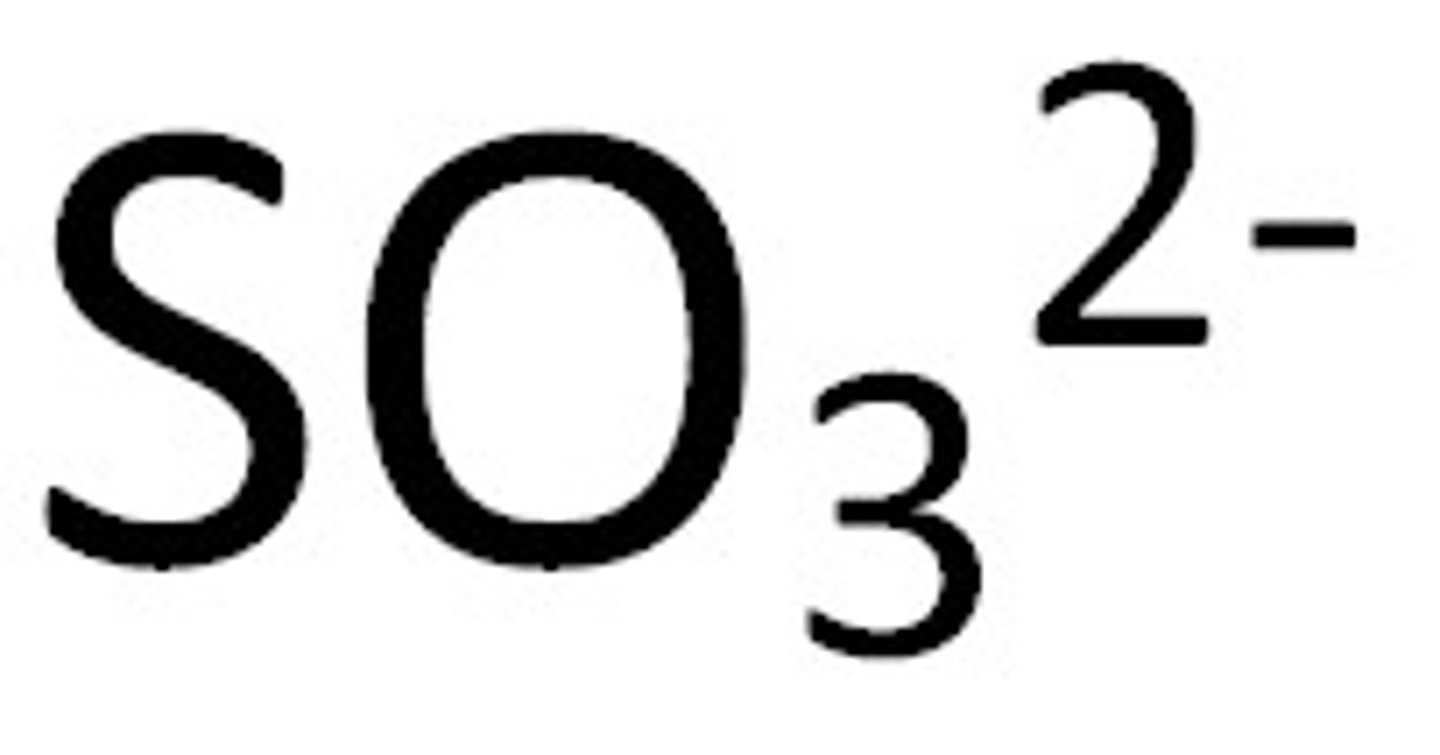
hydrogen carbonate (or bi carbonate)
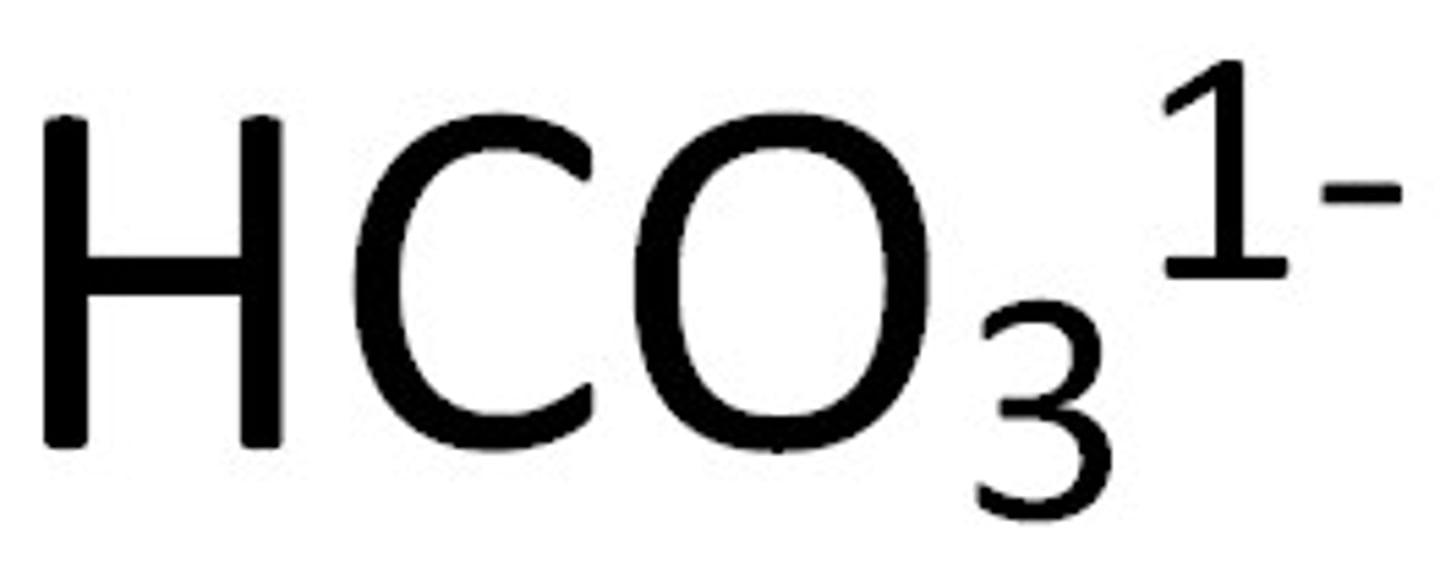
hypochlorite
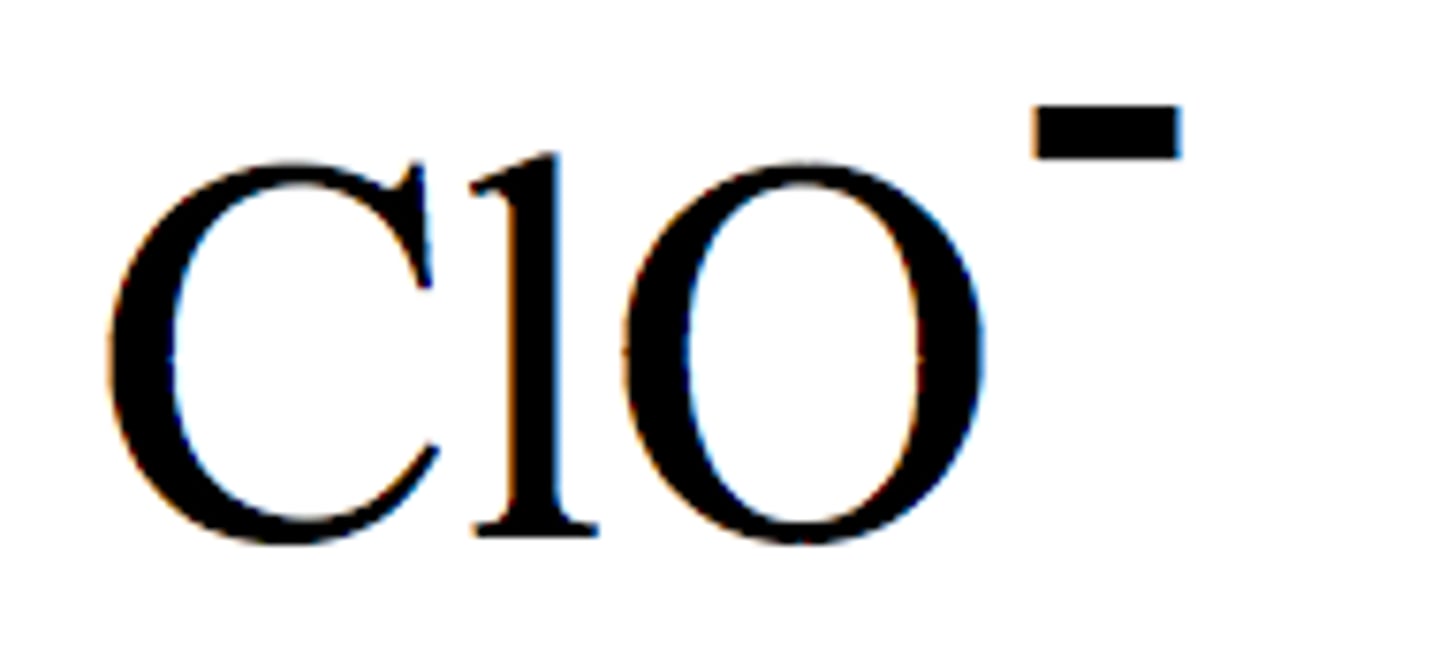
sulfate
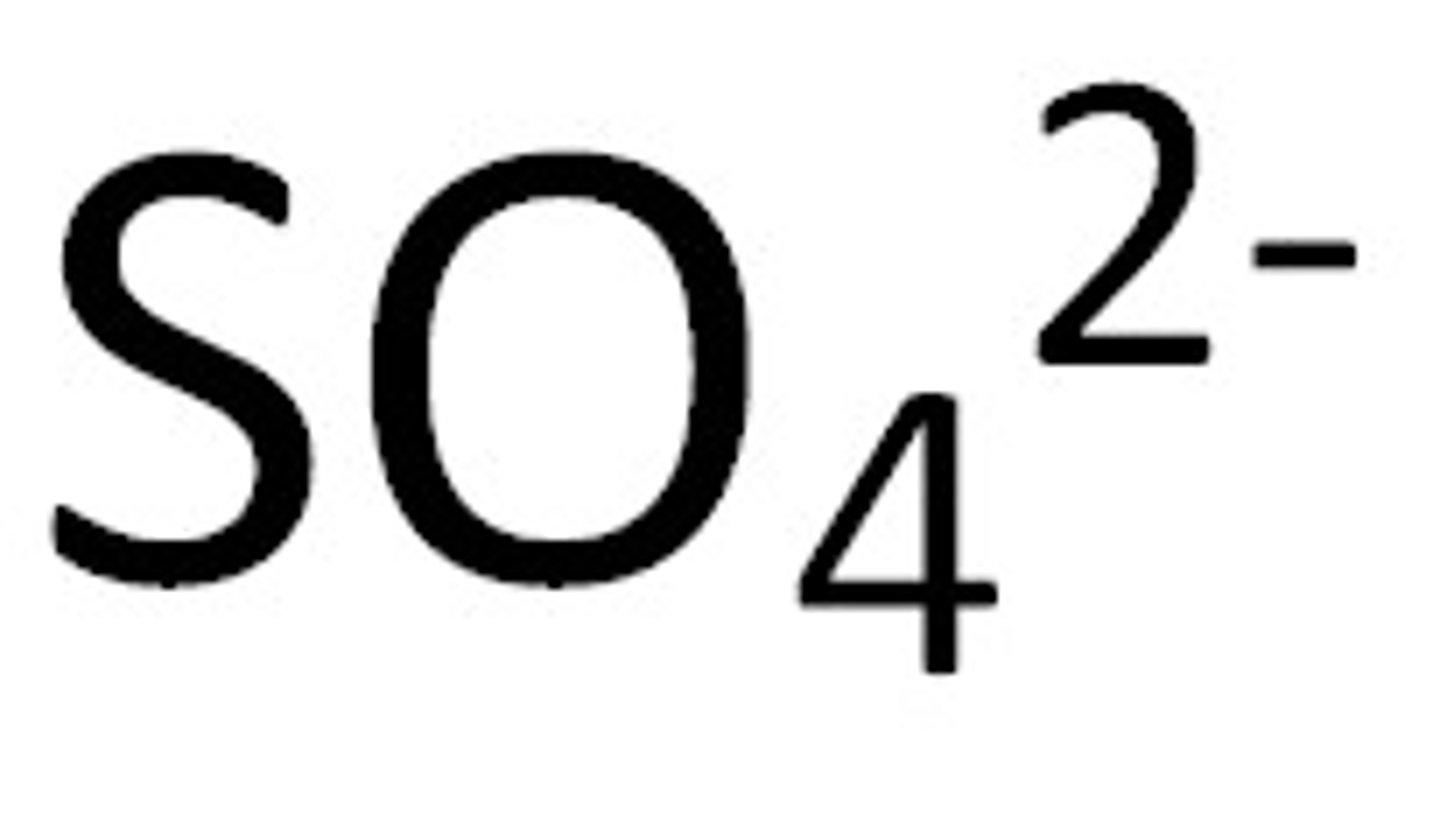
chlorite
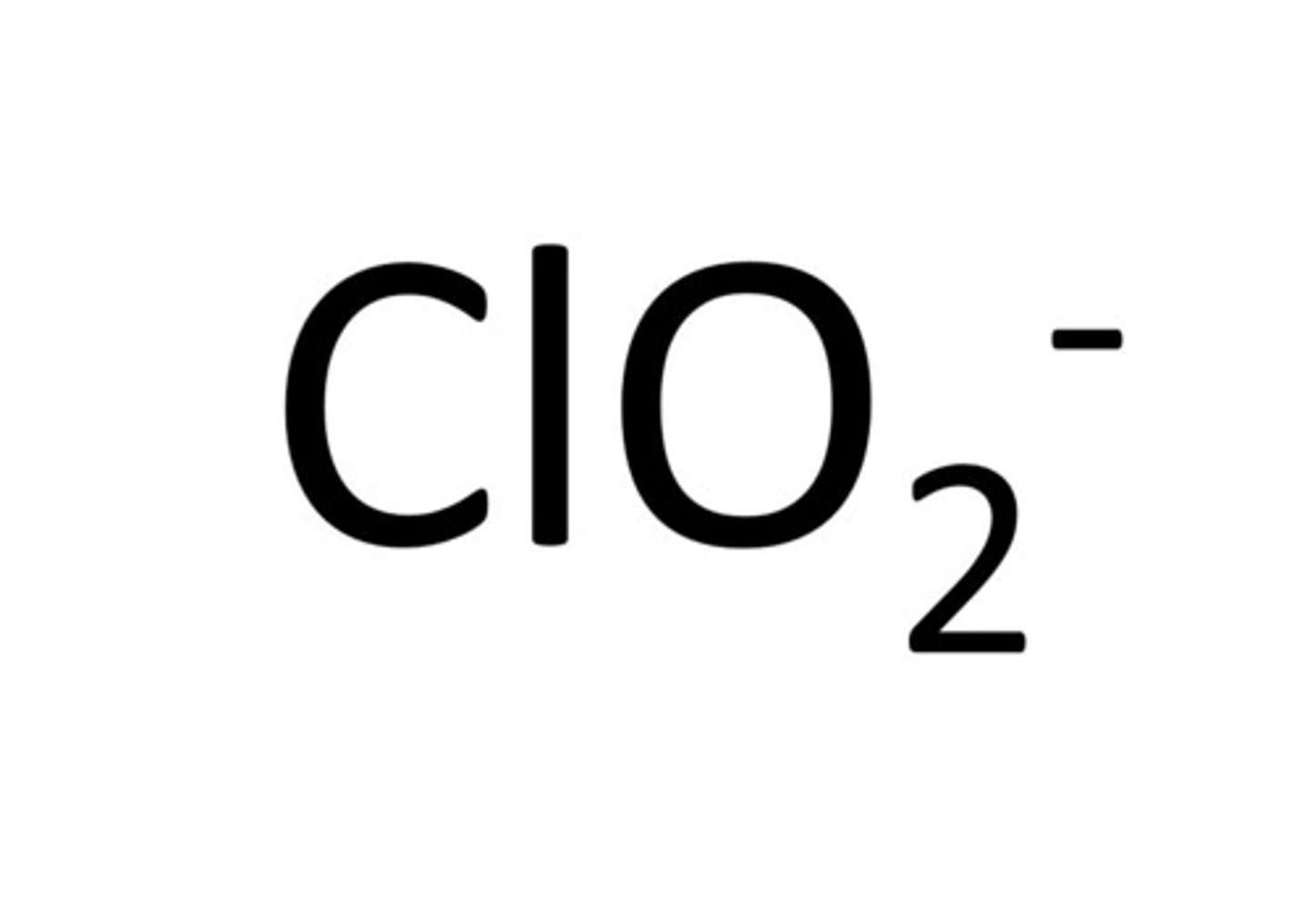
perchlorate
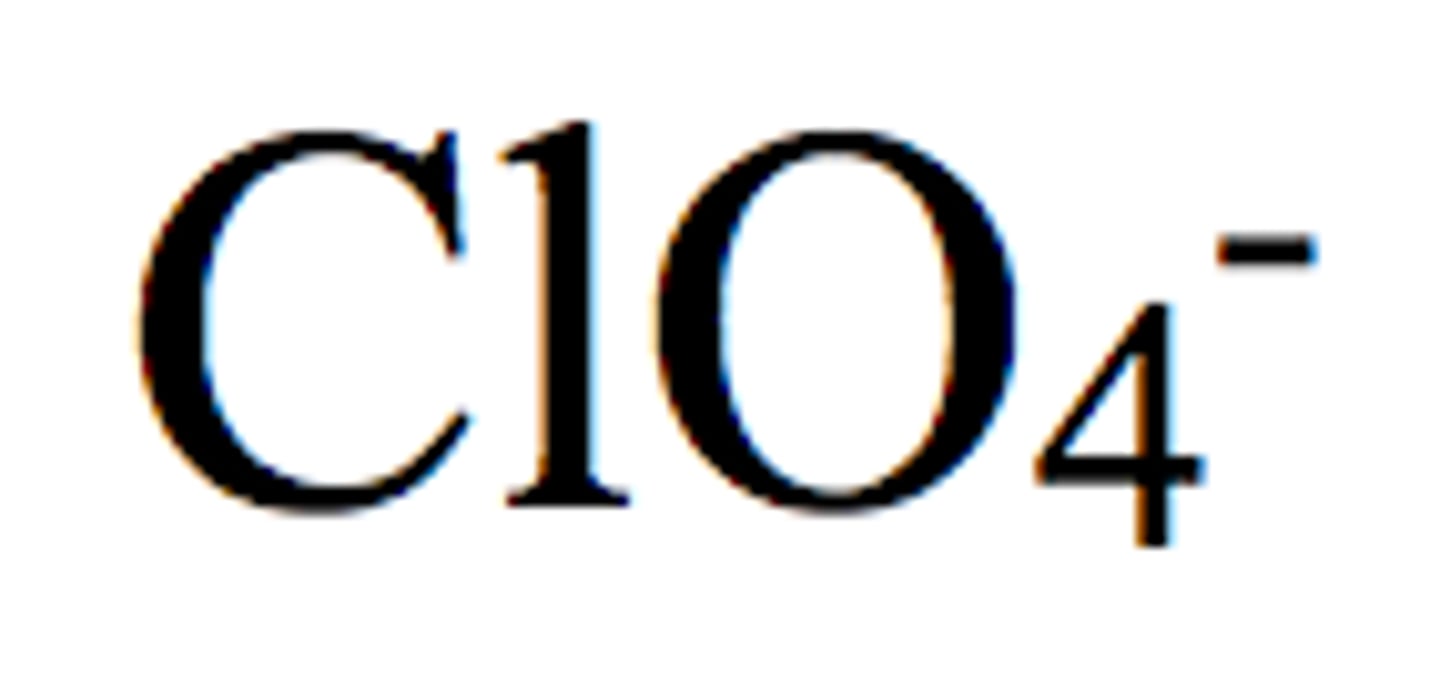
Oxalate Ion
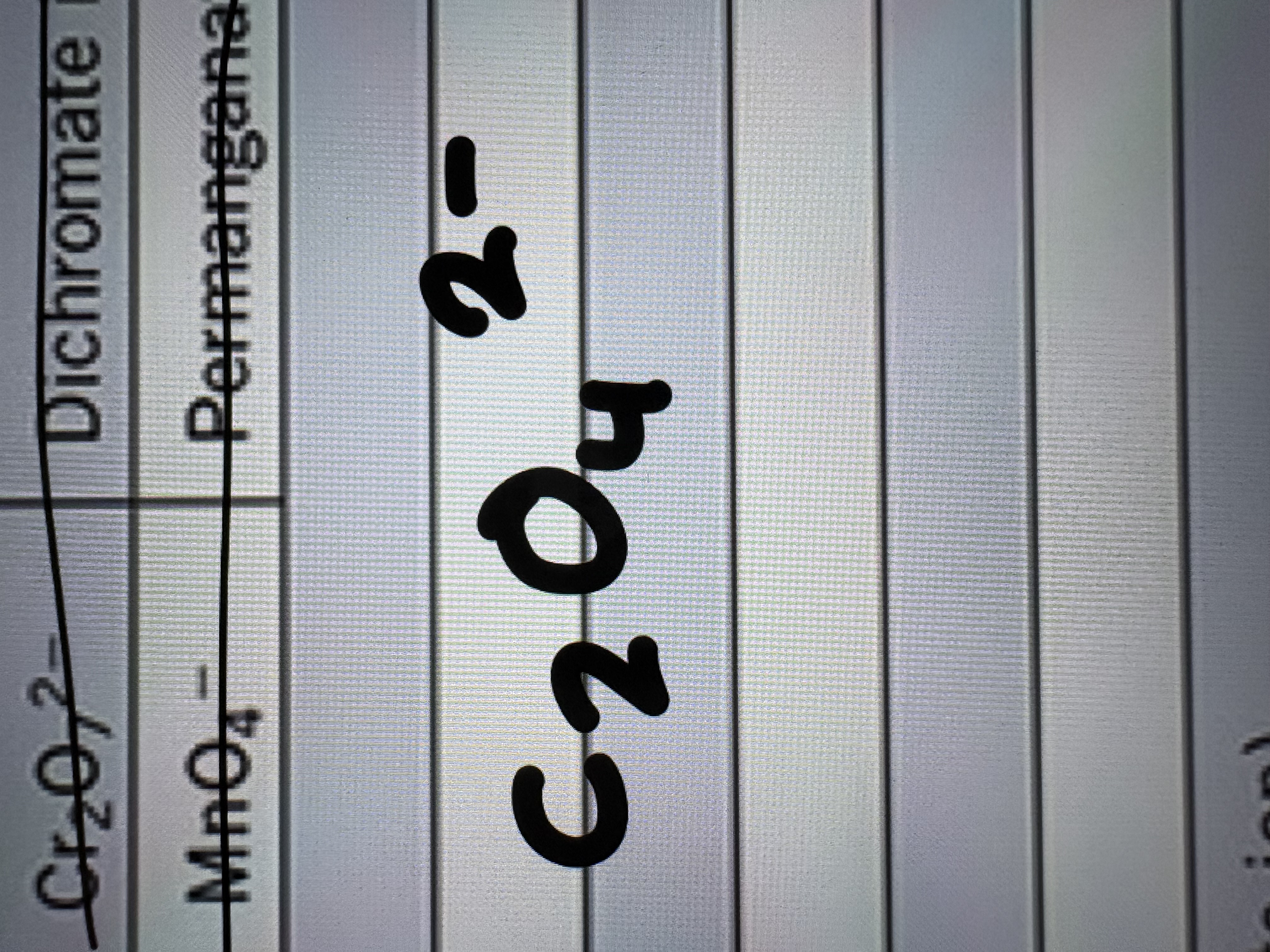
Hydrogen Phosphate Ion
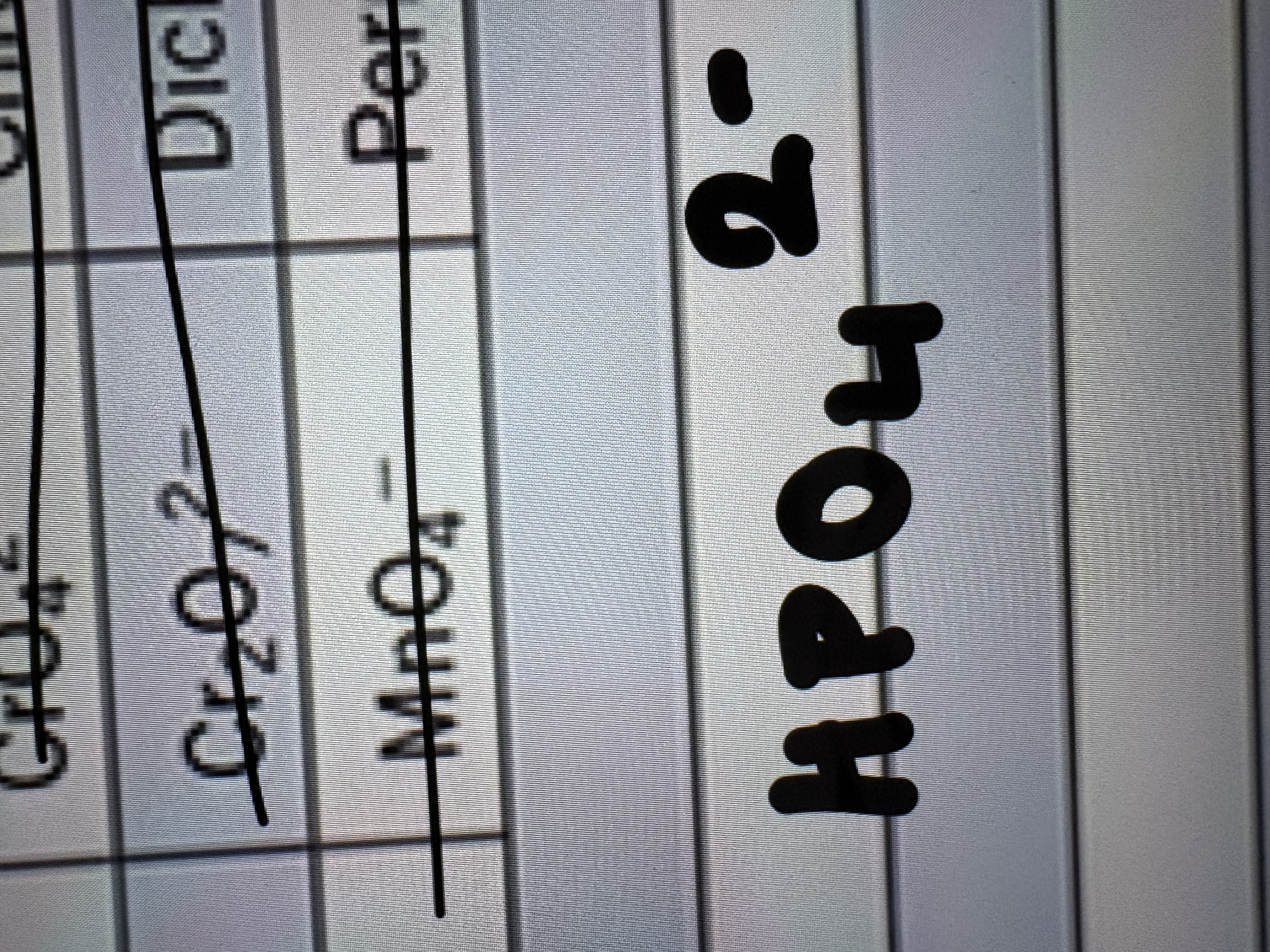
Dihydrogen Phosphate Ion
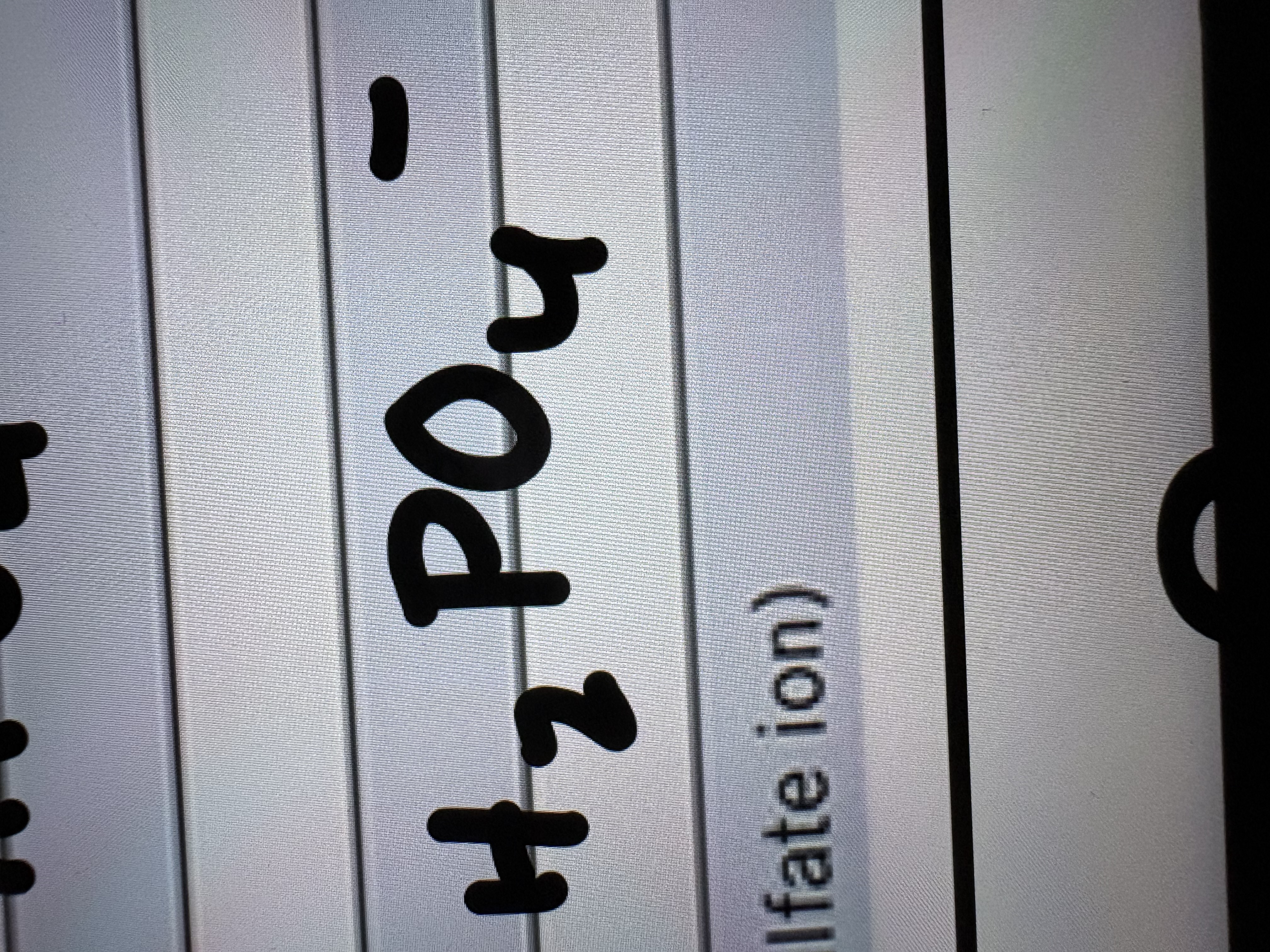
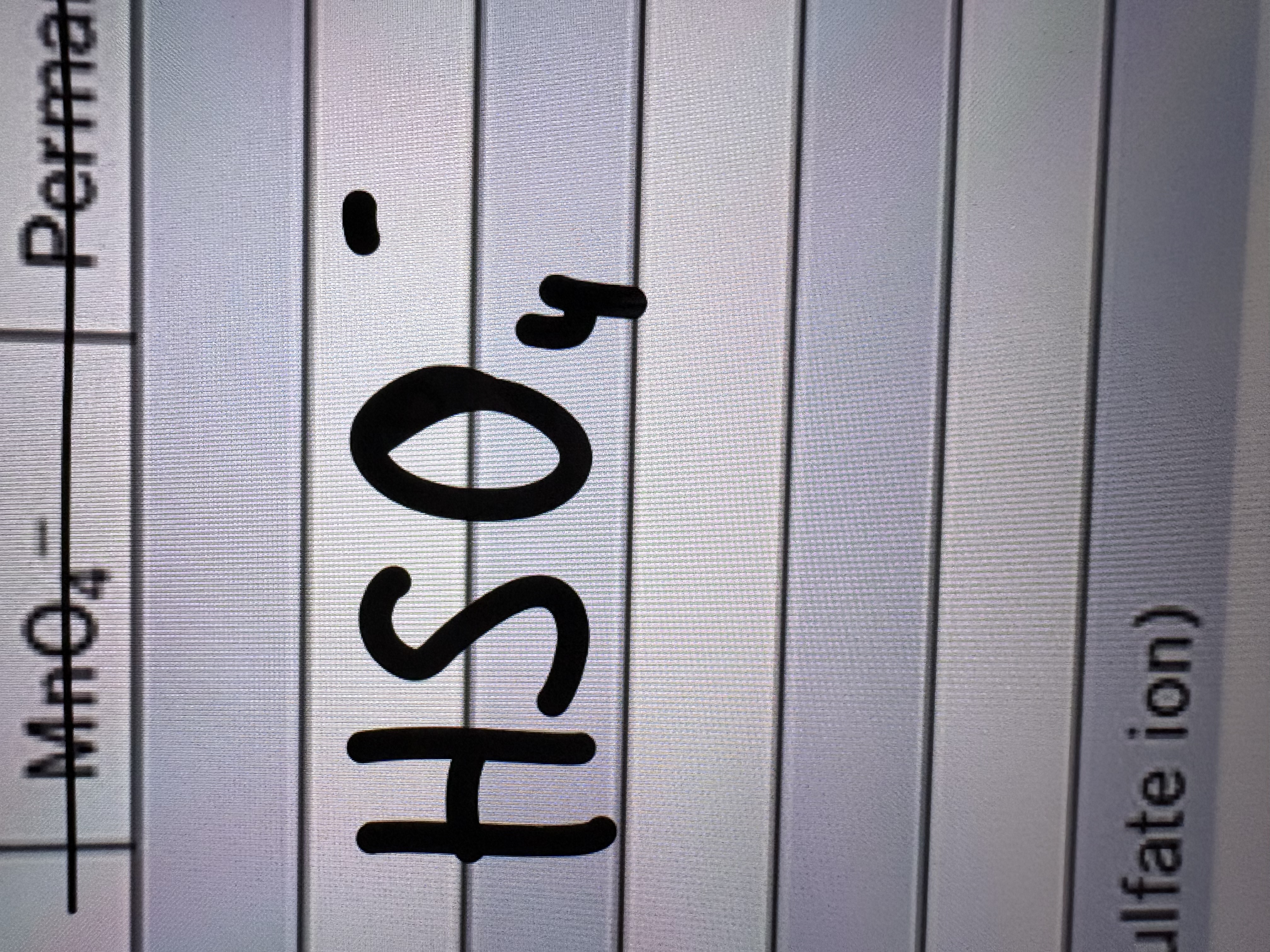
Hydrogen Sulfate Ion (or Bi Sulfate)
Hypobromite

Bromite
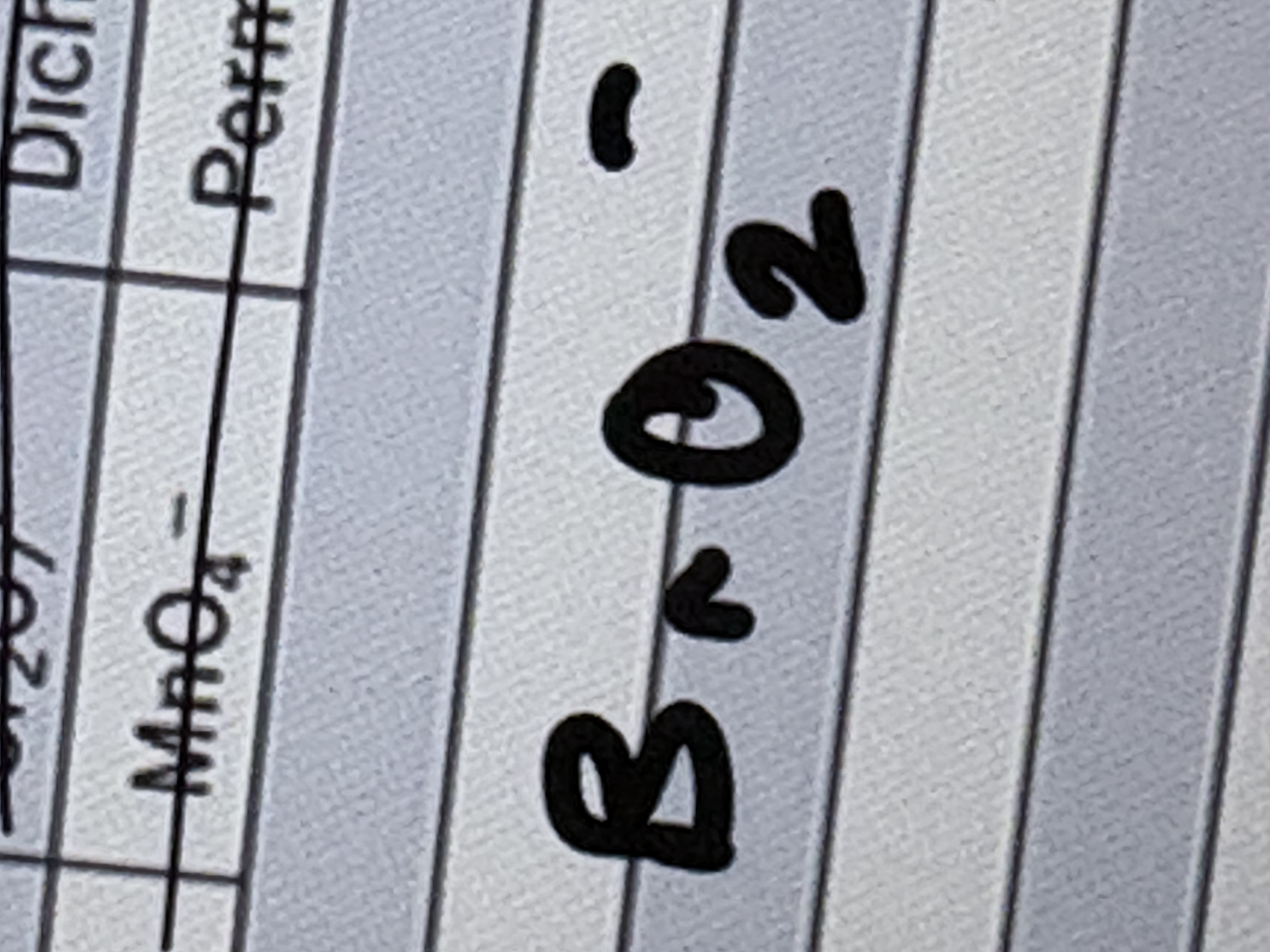
Bromate
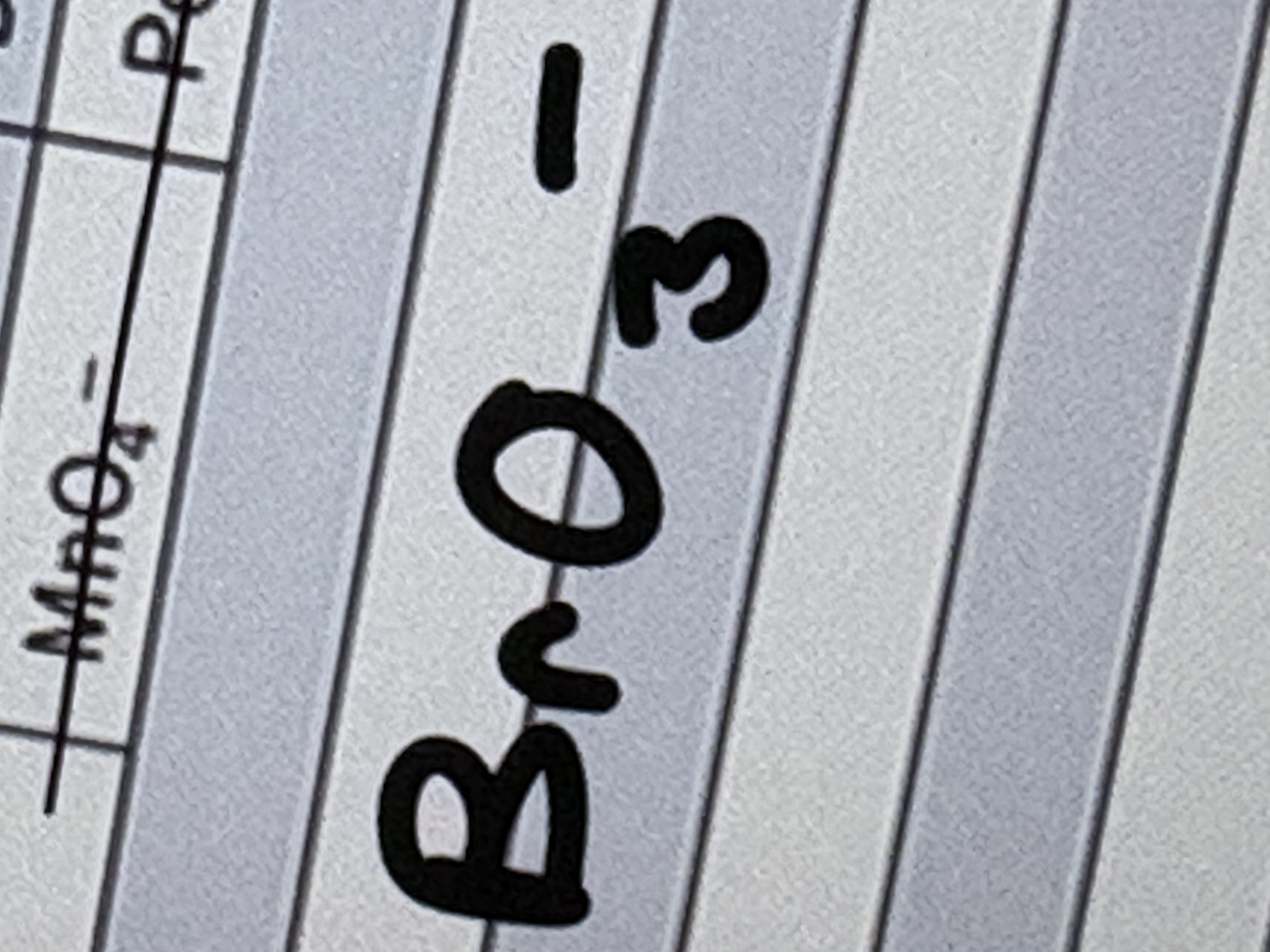
Perbromate

Hypoiodite

Iodite

Iodate

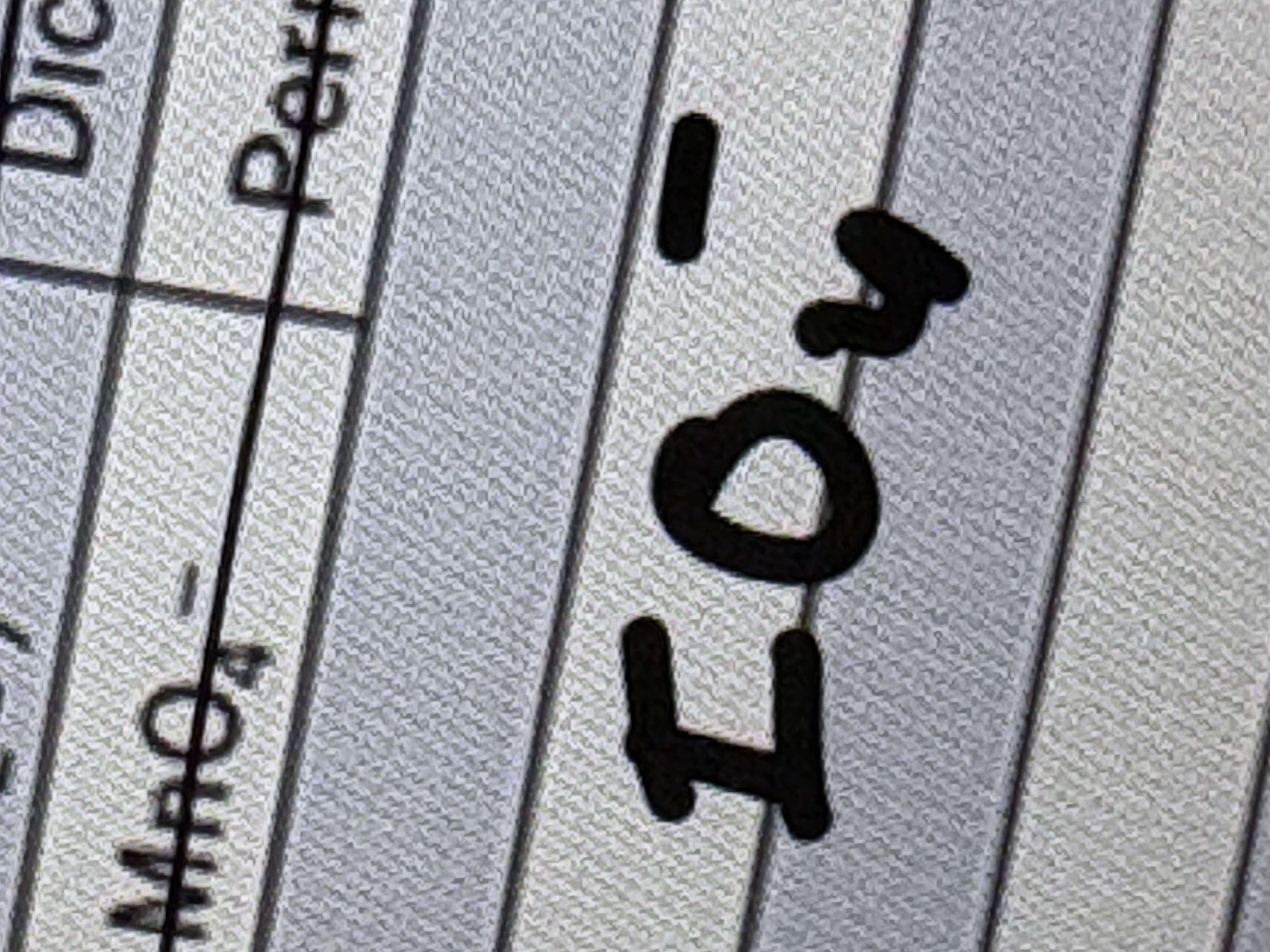
Periodate
lithium
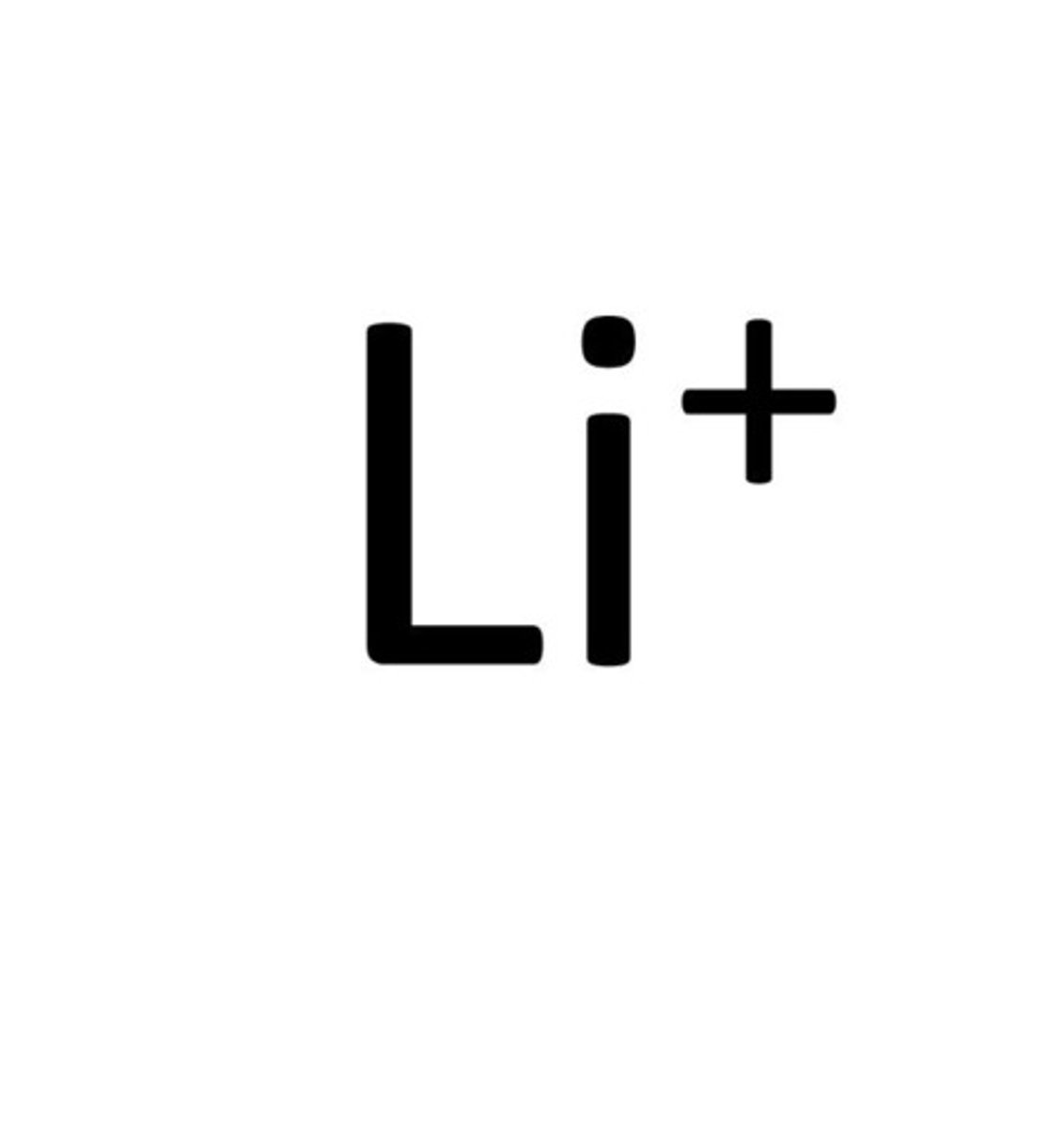
nitrogen
N3-
oxygen
O-2
halogens
F, Cl, I,Br :-1
carbon
SKIPPP
sodium
Na+1
magnesium
Mg+2
calcium
Ca +2
silver
Ag+1
Fe
SKIPPPP
Cu
Copper +1 or +2
Zinc
Zn+2
Sulfur
S -2
Lead
SKIPPP
Au
SKIPPPP
Phosphorus
P - 3
Sn
SKIPPPP
Ba
barium +2
Strontium
Sr+2
Hydrogen
H +1
Noble Gases and Charge?
He, Ar, Ne, Kr, Xe, Rn, Og || No charge
How to name Ionic Compounds: Fixed Charge?
Cation + (base anion + -ide)
Alkali Metal’s Group and Charge?
Group 1 || +1
Alkaline Earth Metals
Group 2 || +2
Aluminum
Al 3+
Gallium
Ga 3+
Silver
Ag 1+
Zinc
Zn 2+
Cadmium
Cd 2+
Fixed Charge Cations? (Dont write charge in parentheses)
Alkali Metals +1, Alkaline Earth Metals +2, Al +3, Ga +3, Ag +1, Zn 2+, Cd 2+
Ionic Compounds: Variant Charge Cations
Cation + (charge of per cation in roman numerals) + base anion -ide
Mn_2 O_3
Manganese(III) Oxide
Mercury(1) Carbonate
Hg2CO3
Lead(2) Phosphate
Pb3(PO4)2
AgClO4
Silver Perchlorate