Amino Acids, Amino Acids MCAT
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
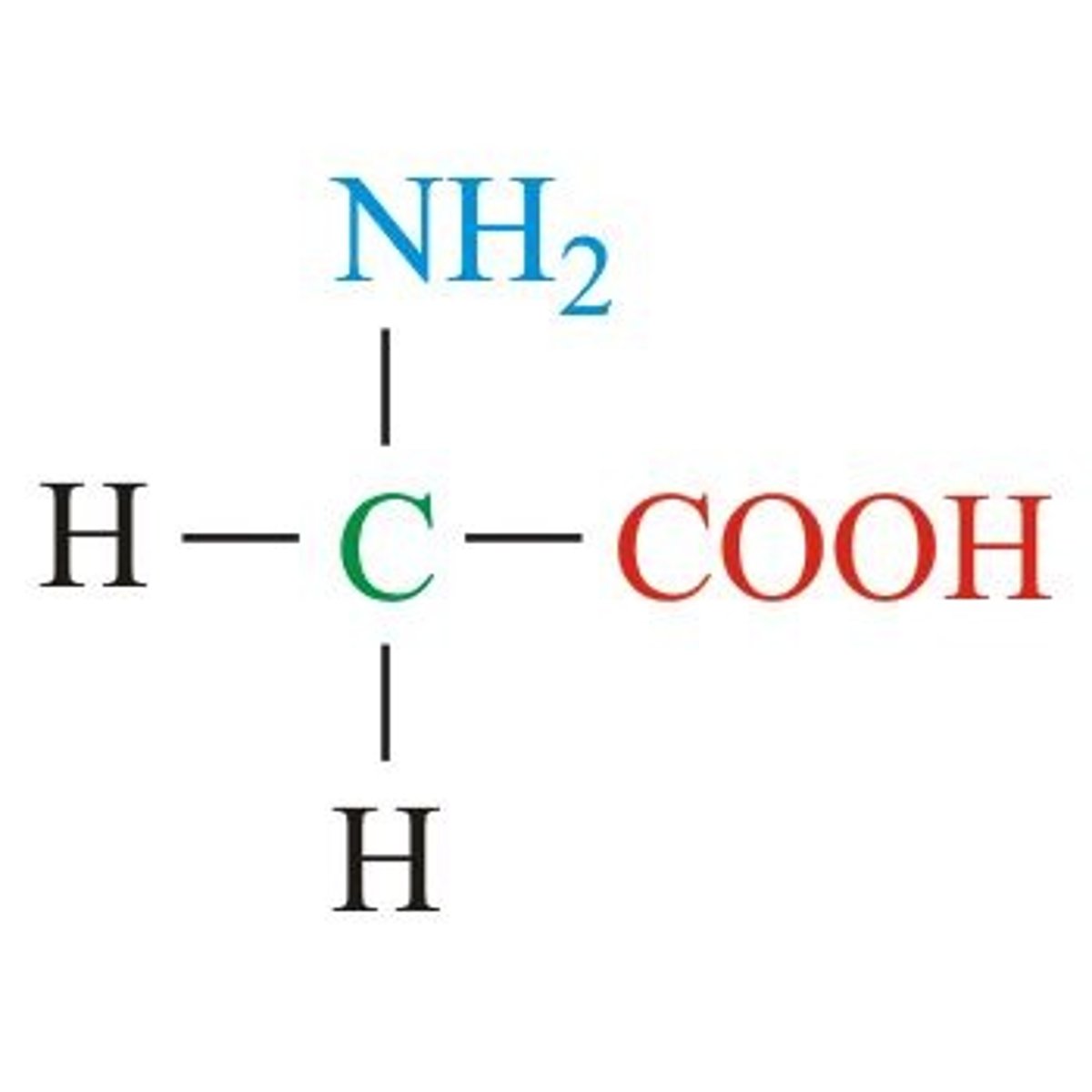
Glycine
Aliphatic (non-polar)
Most simple, optically inactive
Hydrogen for R
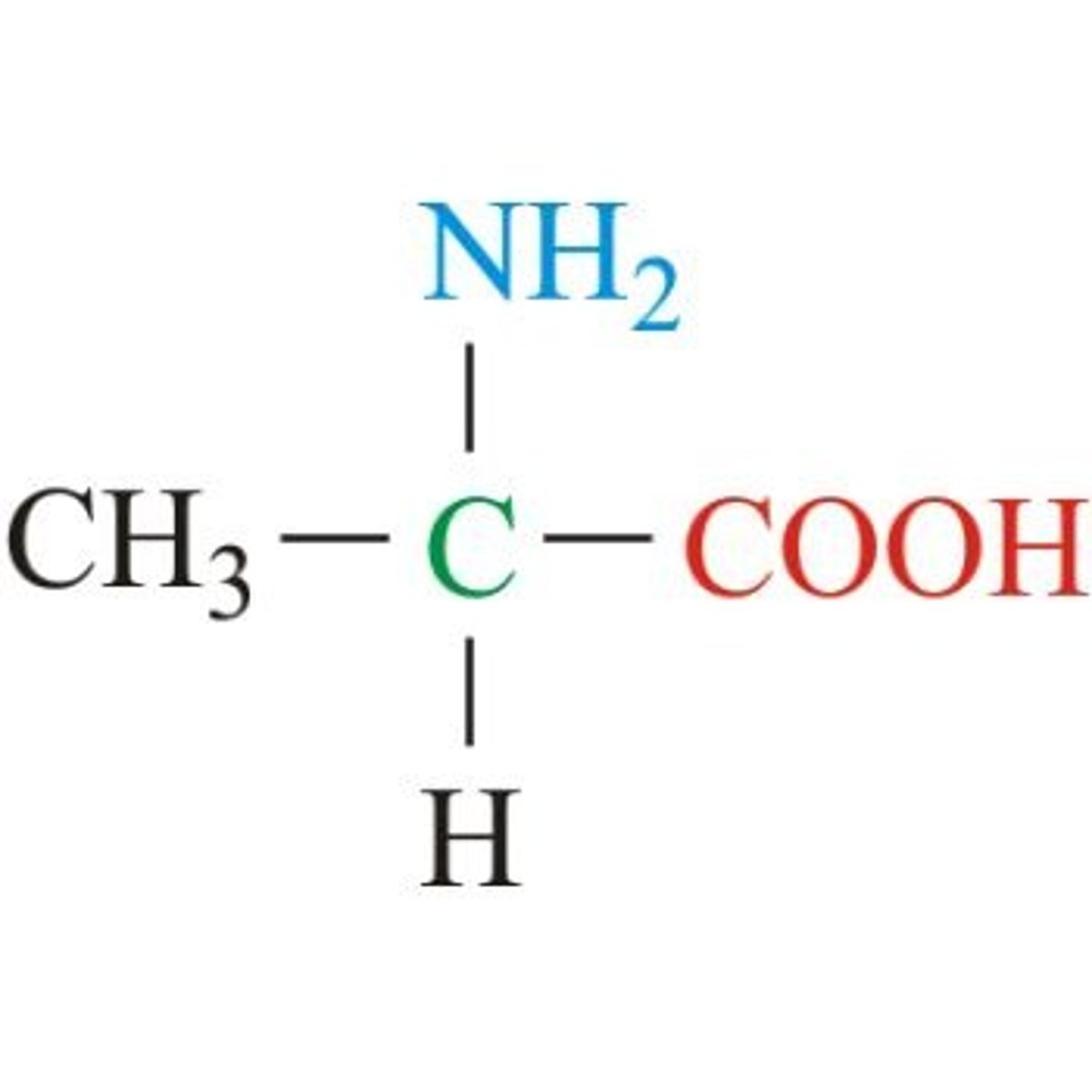
Alanine
Aliphatic (non-polar)
Methyl for R, a simple functional group to start just like "A" starts alphabet
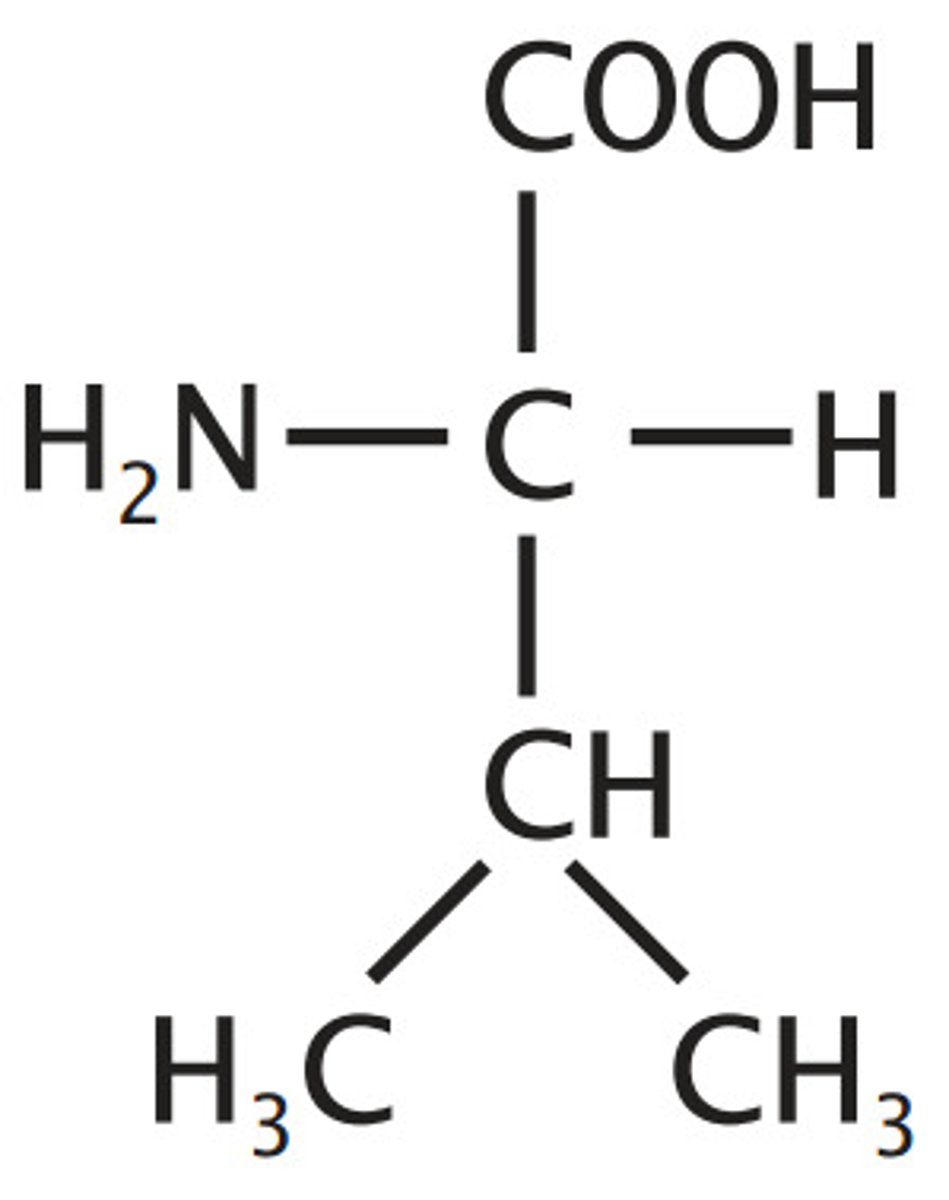
Valine
Aliphatic (non-polar)
Simple, R shaped like a V
Leucine
Aliphatic (non-polar)
Valine extended with one methyle
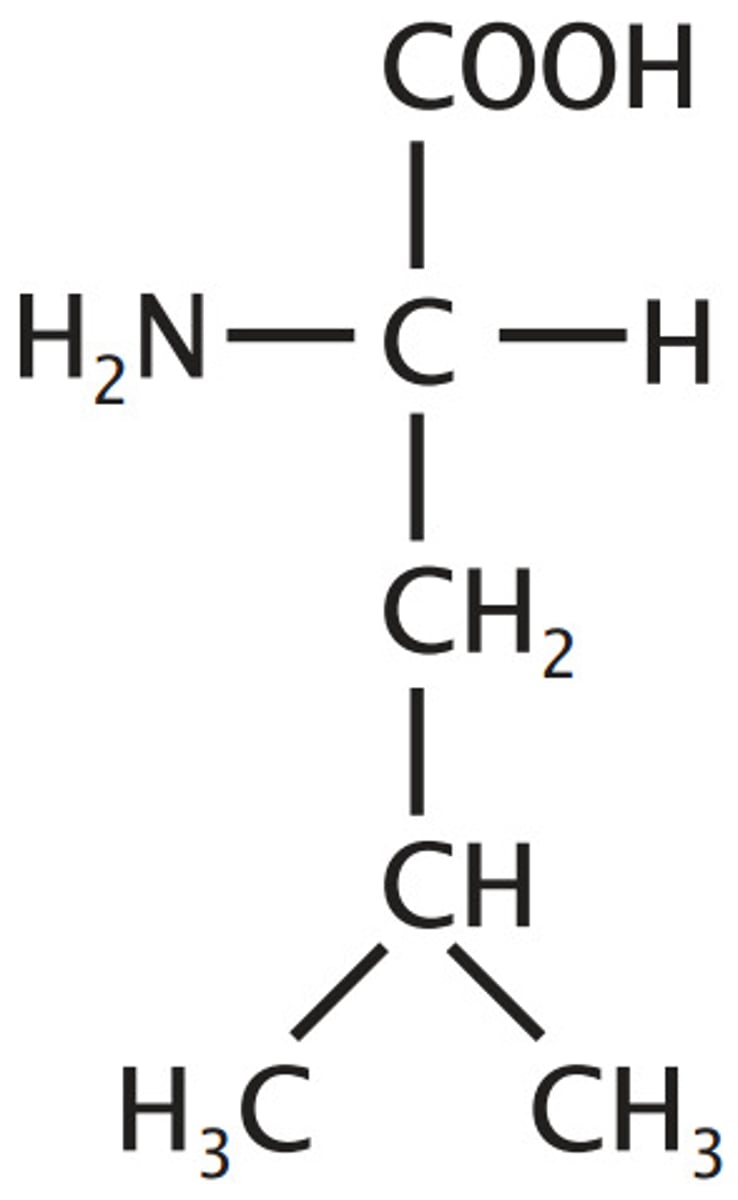
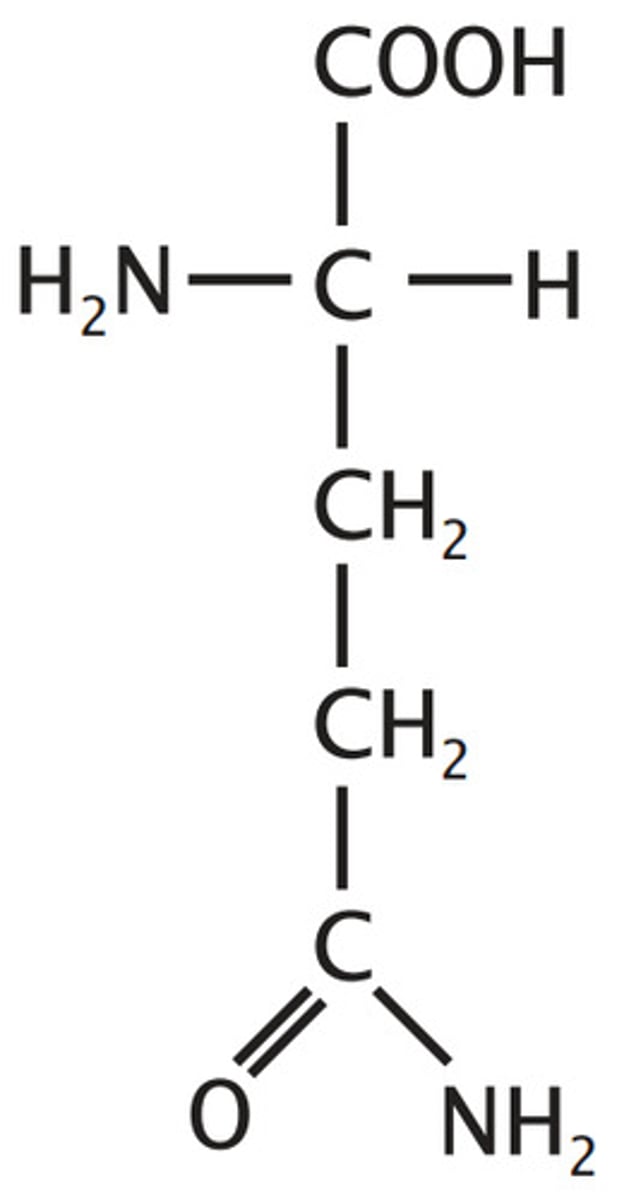
Isoleucine
Aliphatic (non-polar)
"Lopsided Valine"
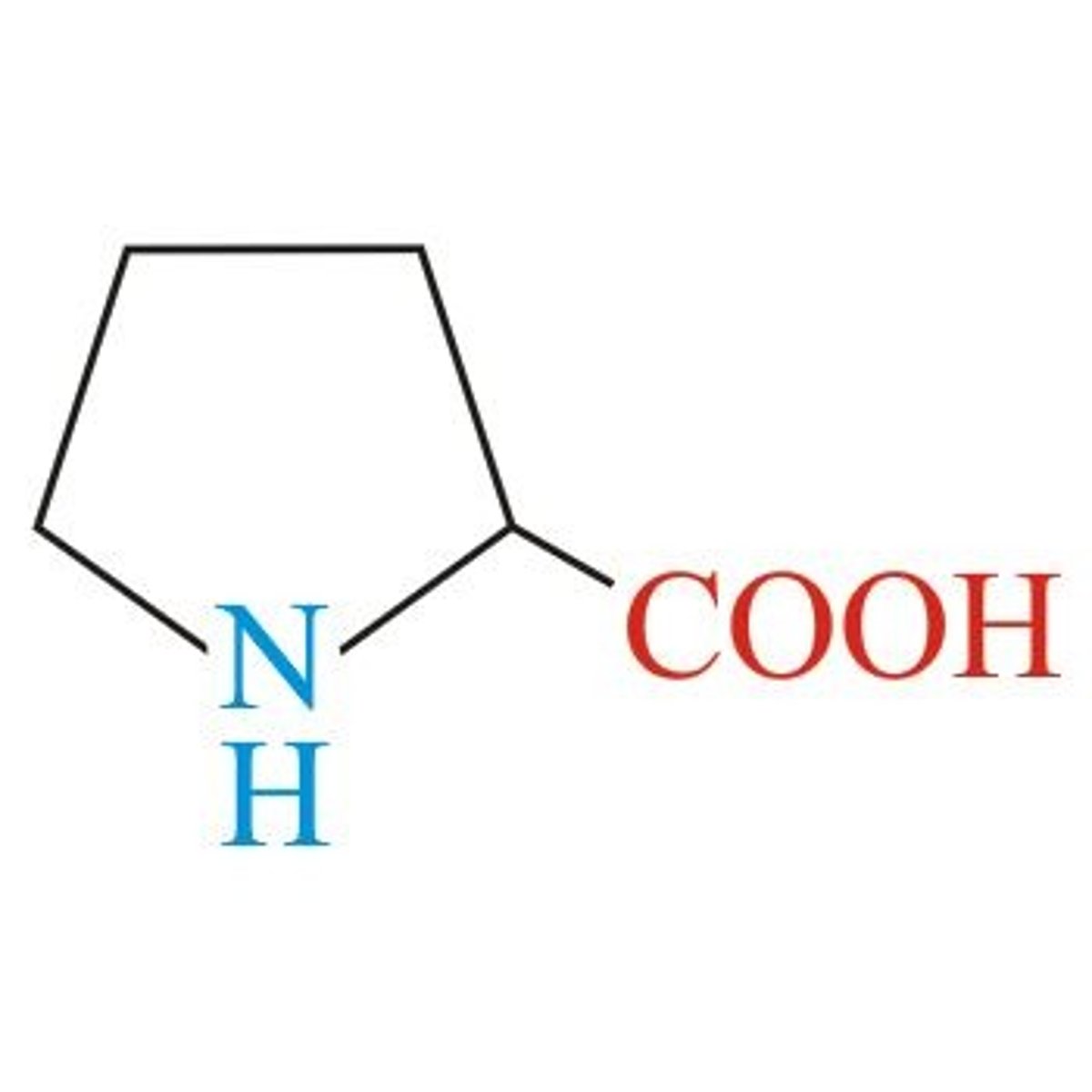
Proline
Aliphatic (non-polar)
3 Carbon chain to N
Special Structure found in turns
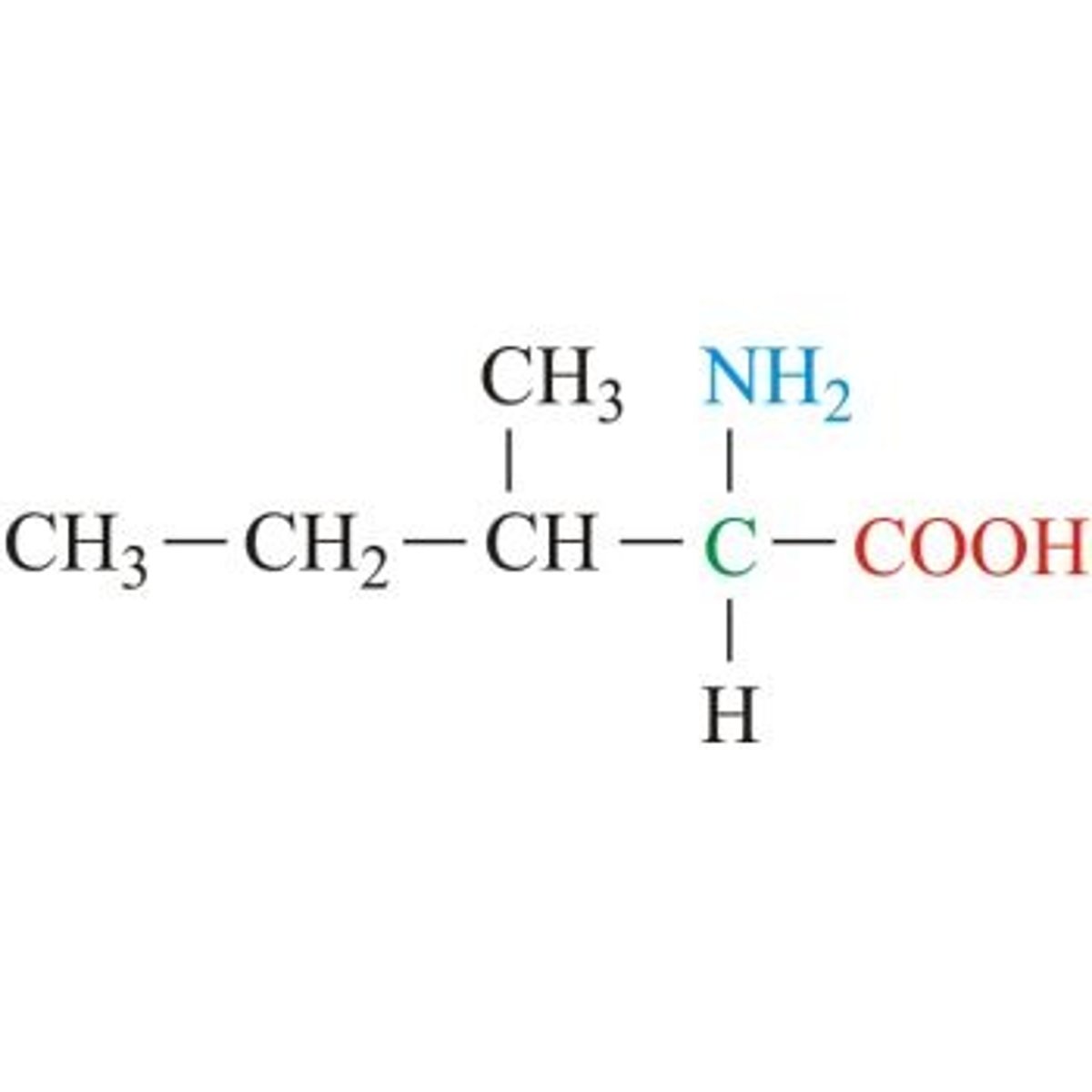
Methionine
Sulfur Containing
Starts every protein
3 Carbons with a thioether
methyl blocked sulfhydryl
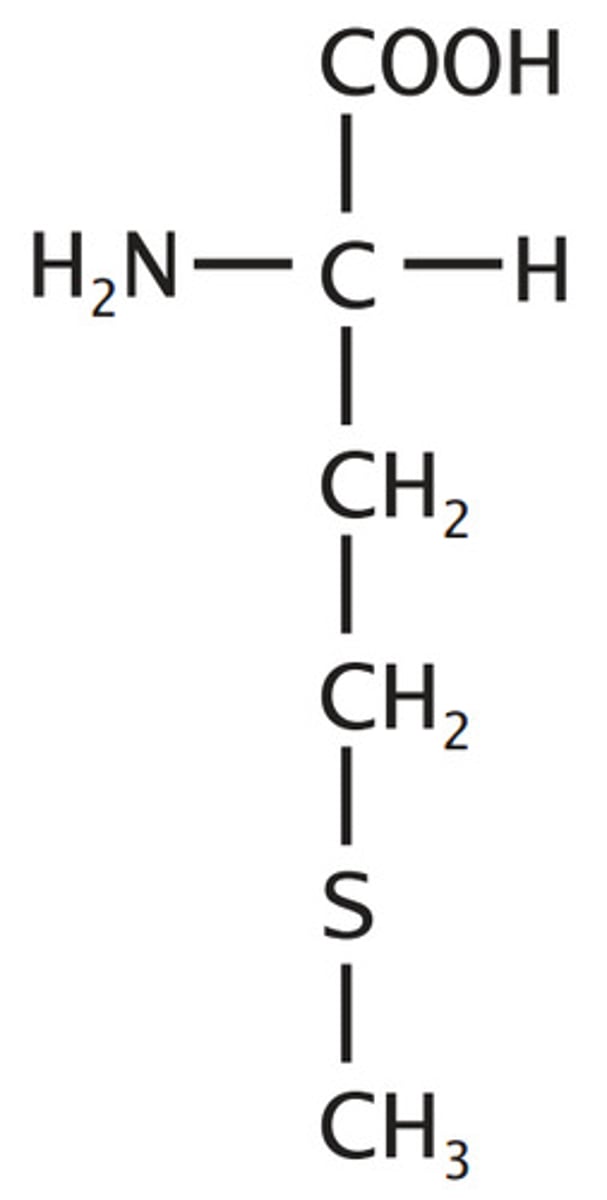
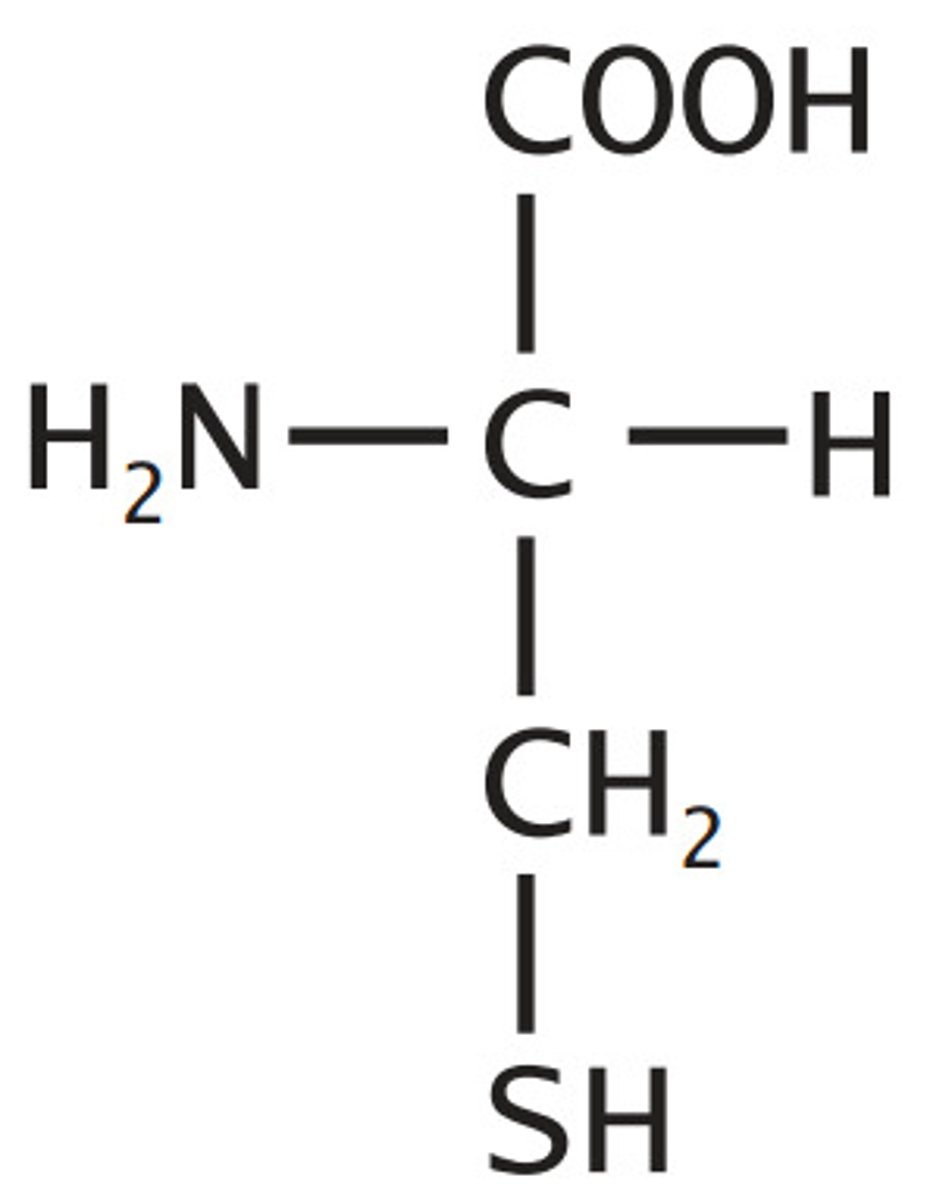
Cysteine
Sulfur Containing
Sulfhydryl alanine
reactive, can form disulfides
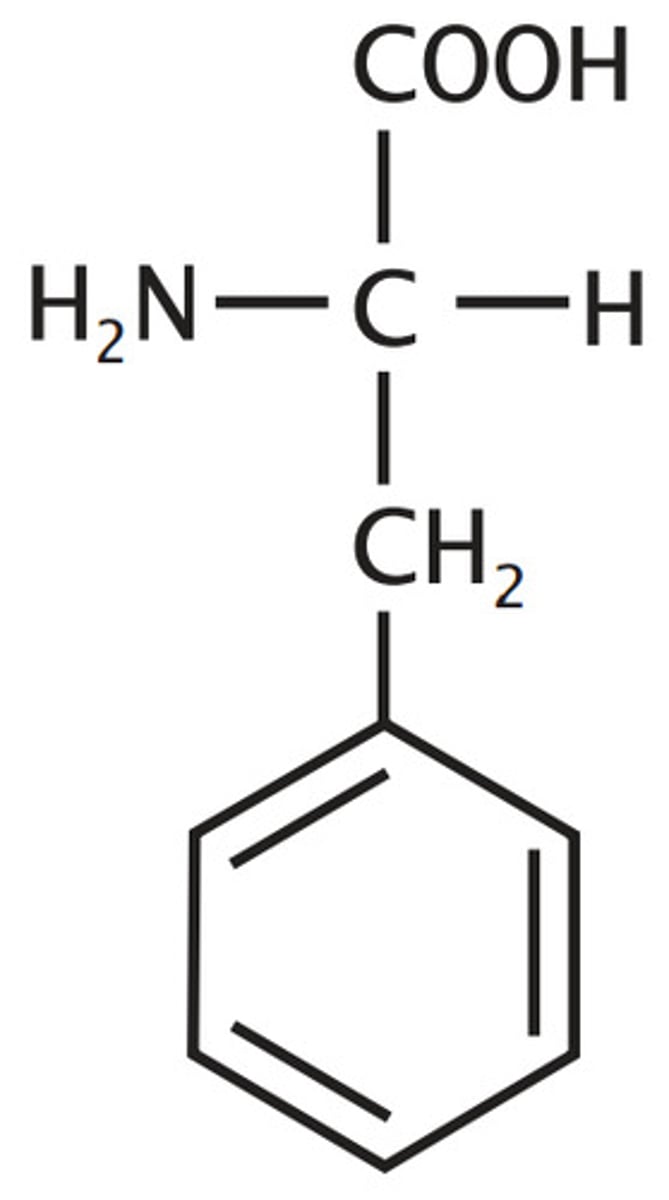
Phenylalanine
Aromatic
Alanine with phenyl group
y reminds of aromatics
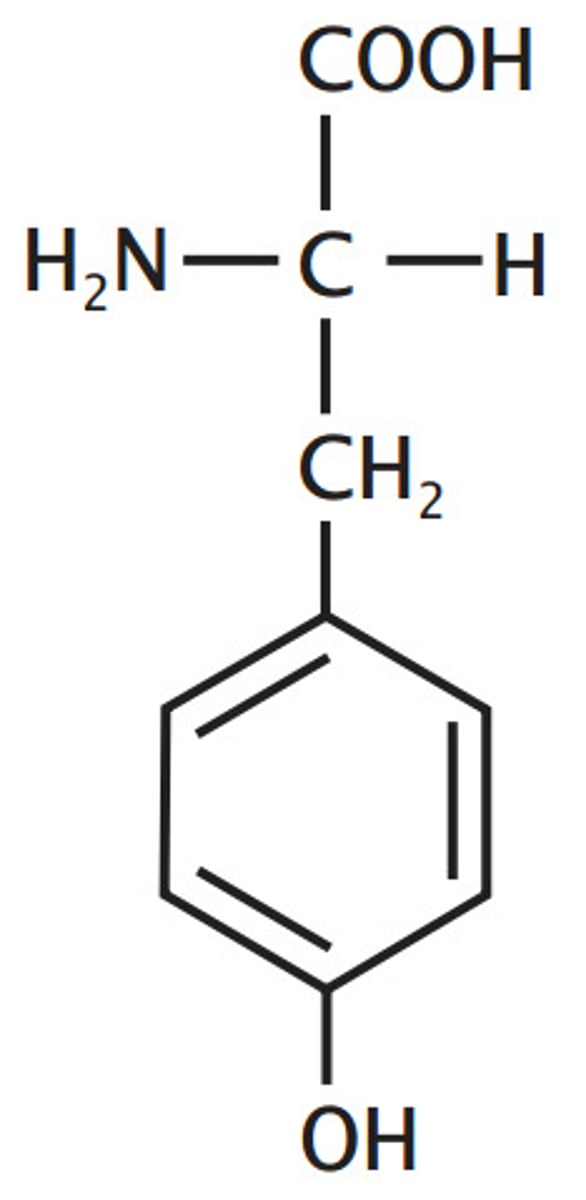
Tyrosine
Aromatic
hydroxylated
phenylalanine, one of 3 "T"s
that has "Y" in its name so it is an aromatic
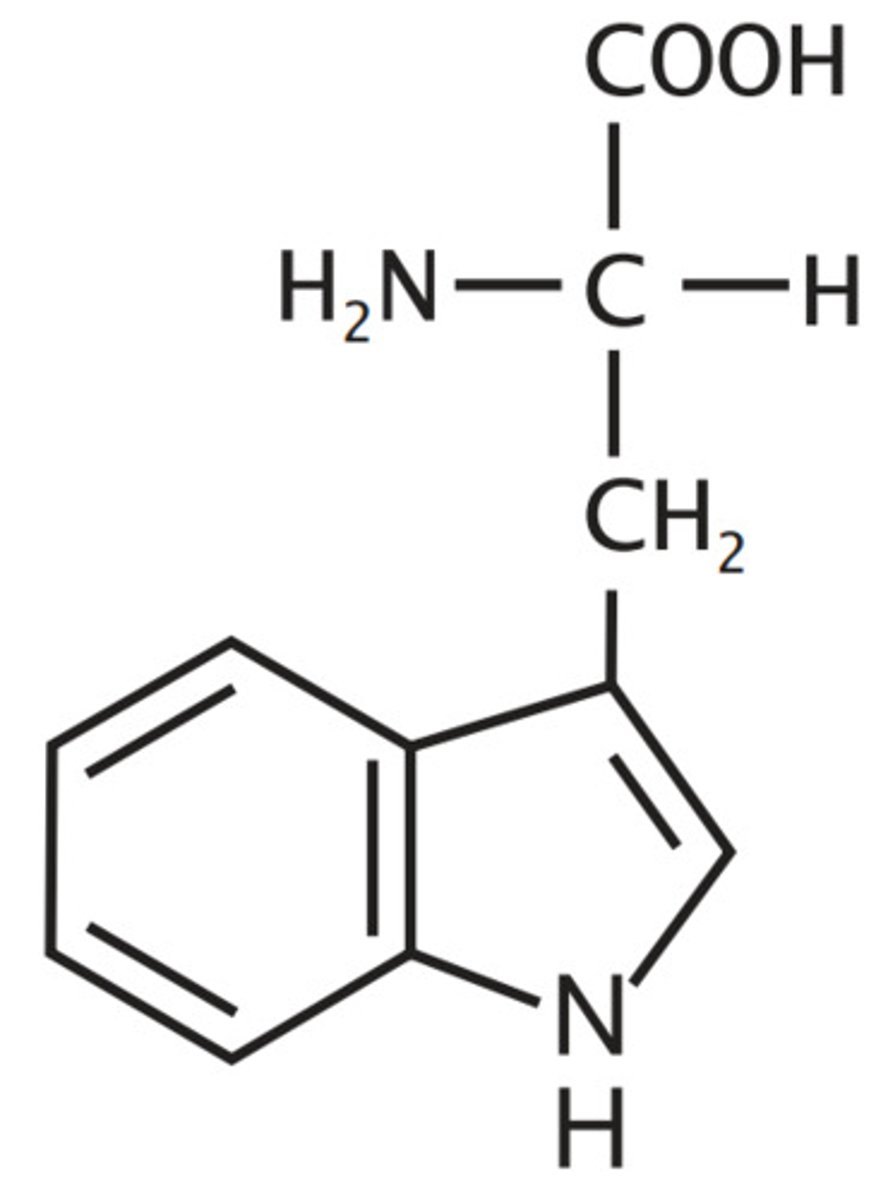
Tryptophan
Aromatic
one of 3 "T"s with a "Y" so it is aromatic, will
"tryp" you up because it is hard to remember,
has a 3 carbon start to N (or indole ring on methylene)
Serine
Aliphatic hydroxyl
"hydroxyl alanine"
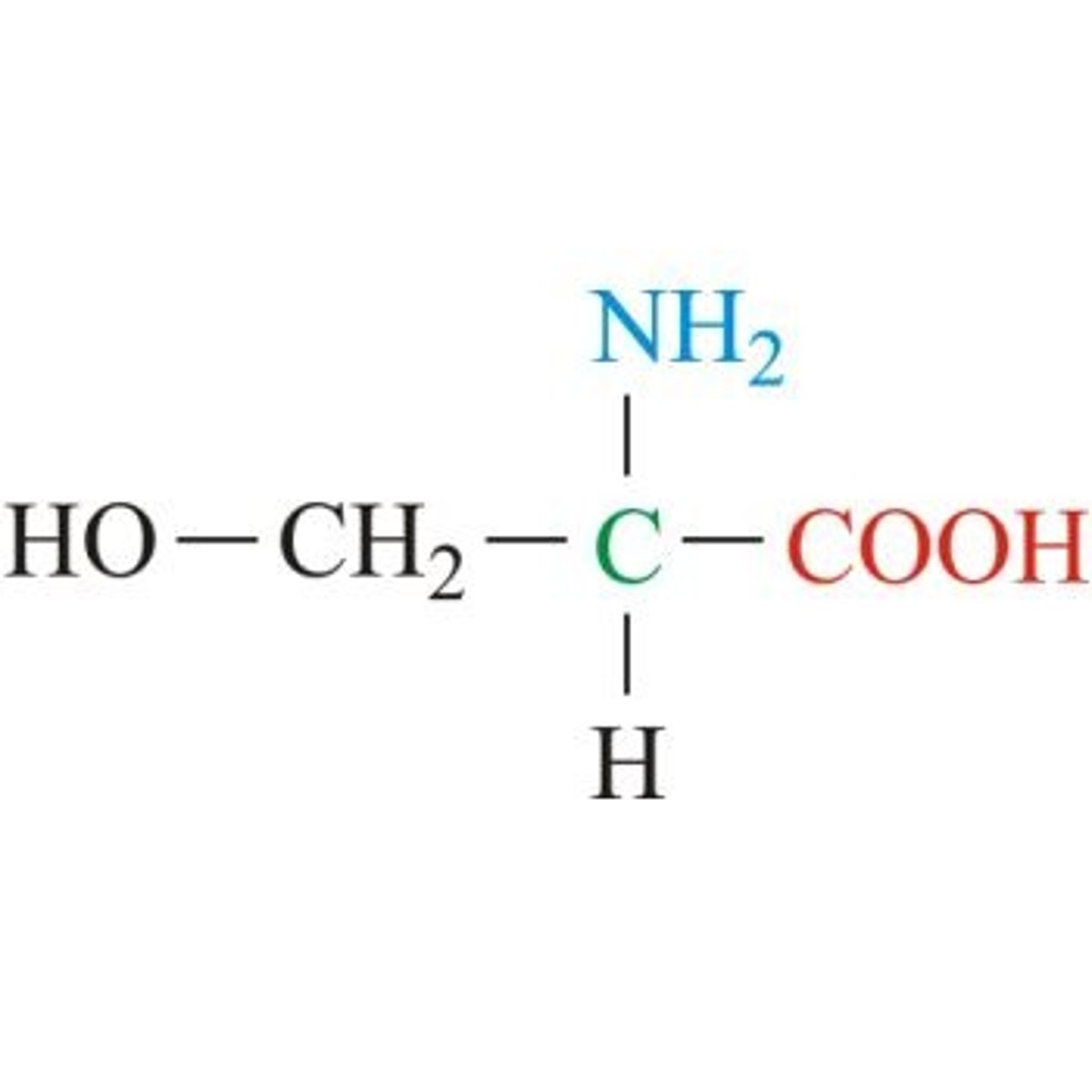
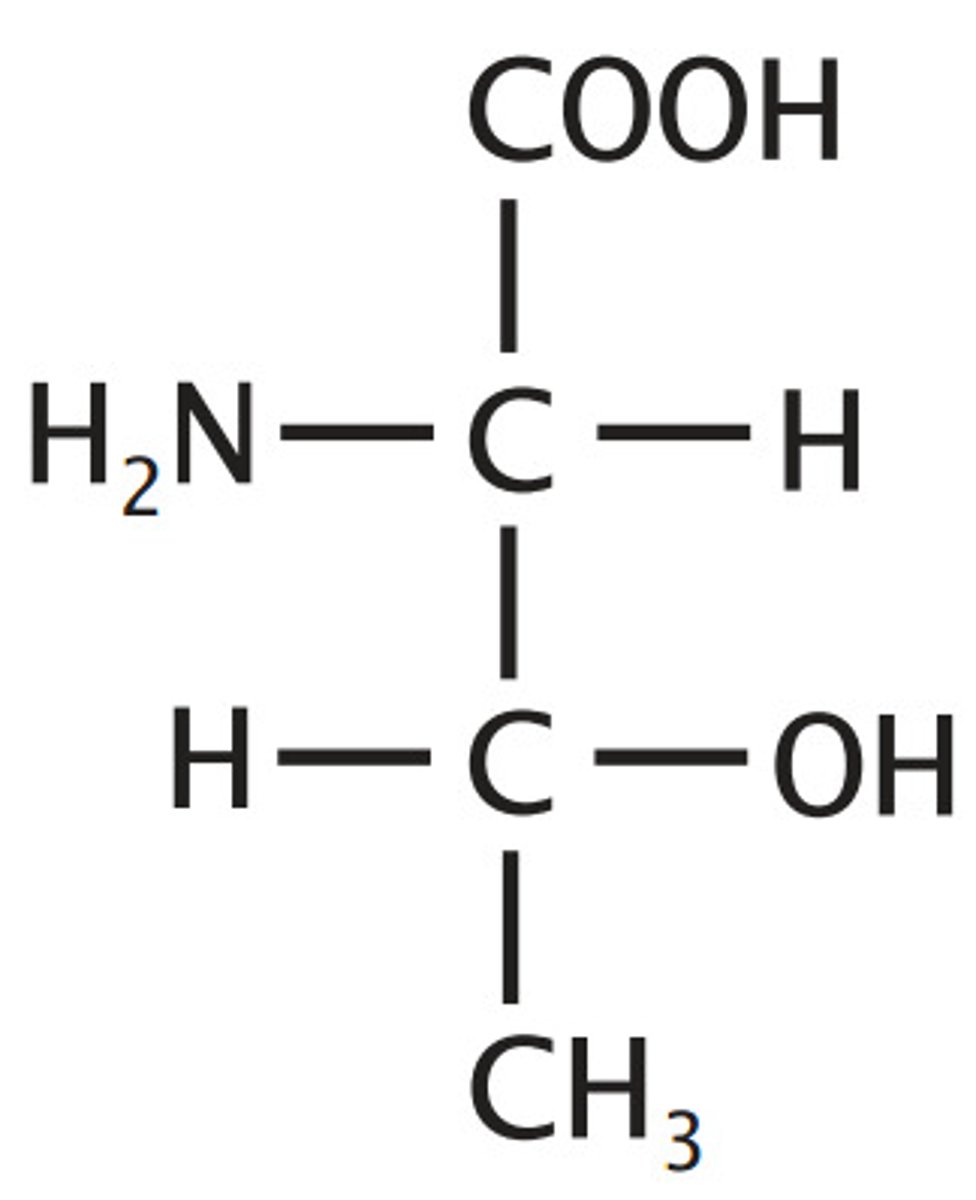
Threonine
Aliphatic, "threo" parts are methyl, hydroxyl, and hydrogen on a single C
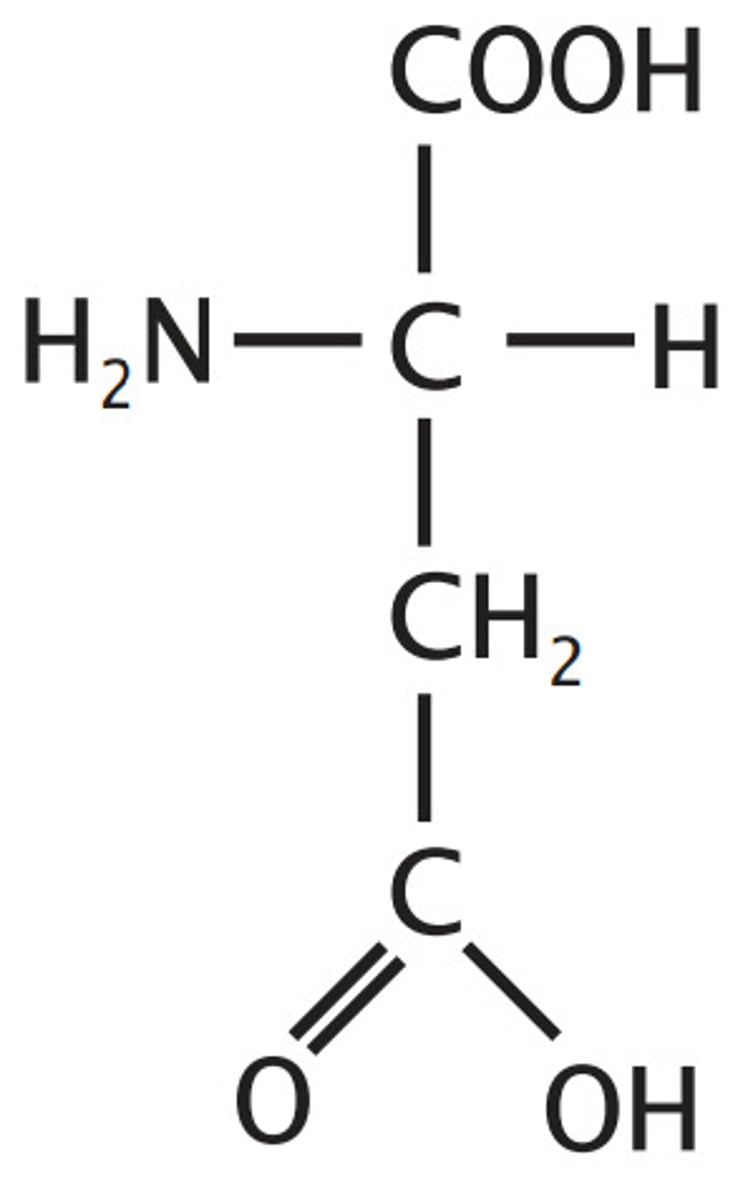
Aspartate
Acidic
"carboxyl alanine"
"ate" -> acidic
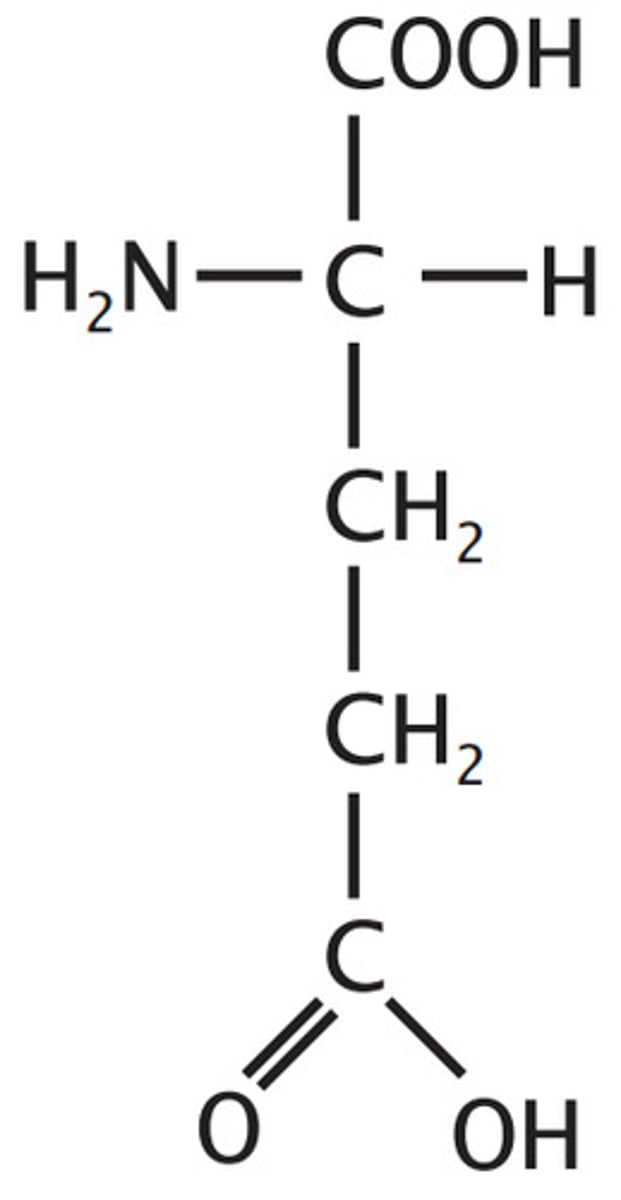
Glutamate
Aspartate plus one methylene, side chain length is signified by alphabetical ordering of the first letter in the names (G is after A)
Arginine
Basic
3 carbon chain linked to a C full of only N's (no H's & C has 4 bonds) through an N
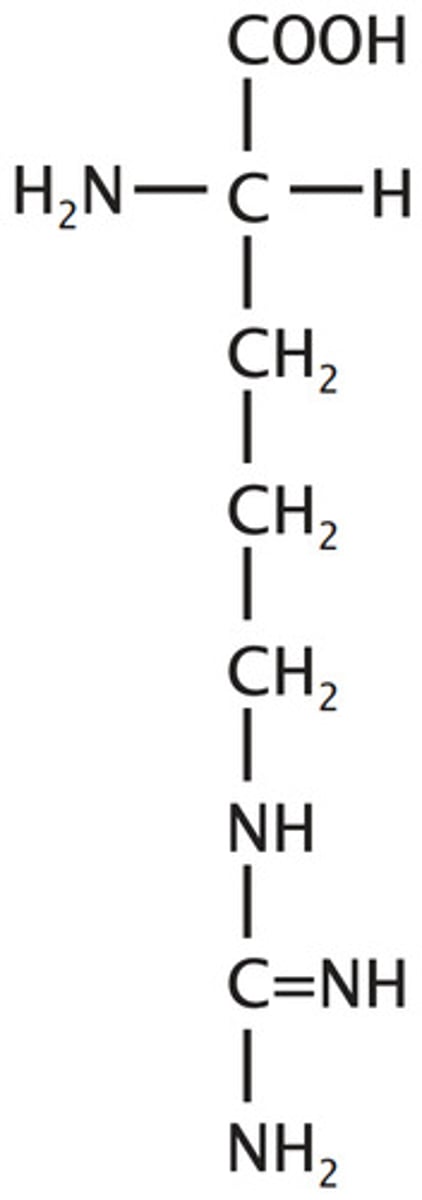
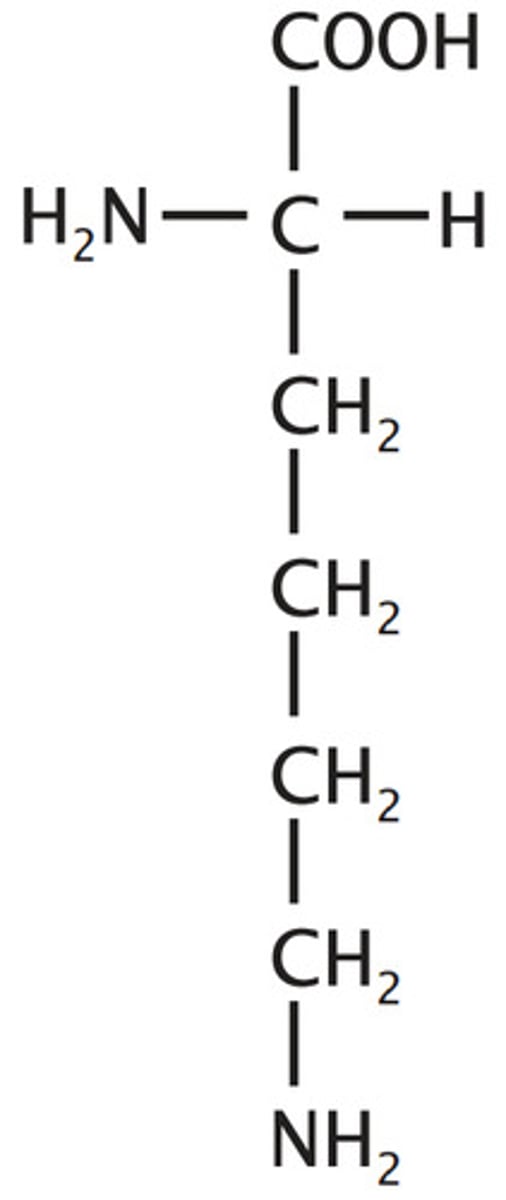
Lysine
Basic
3 carbon chain plus one methylene to amino, it lies ("Lys") about the 3 carbon trend
Histidine
Basic
3 carbons to N and loop back through C 'n' N
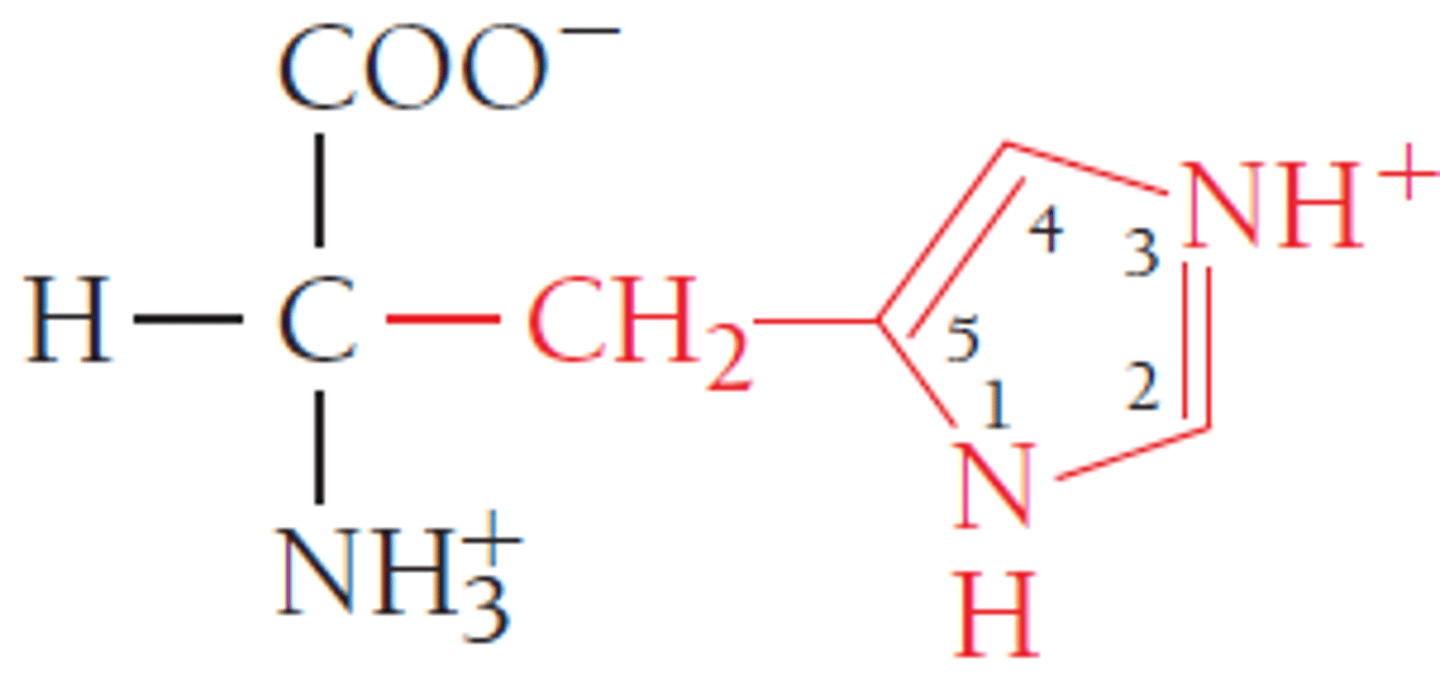
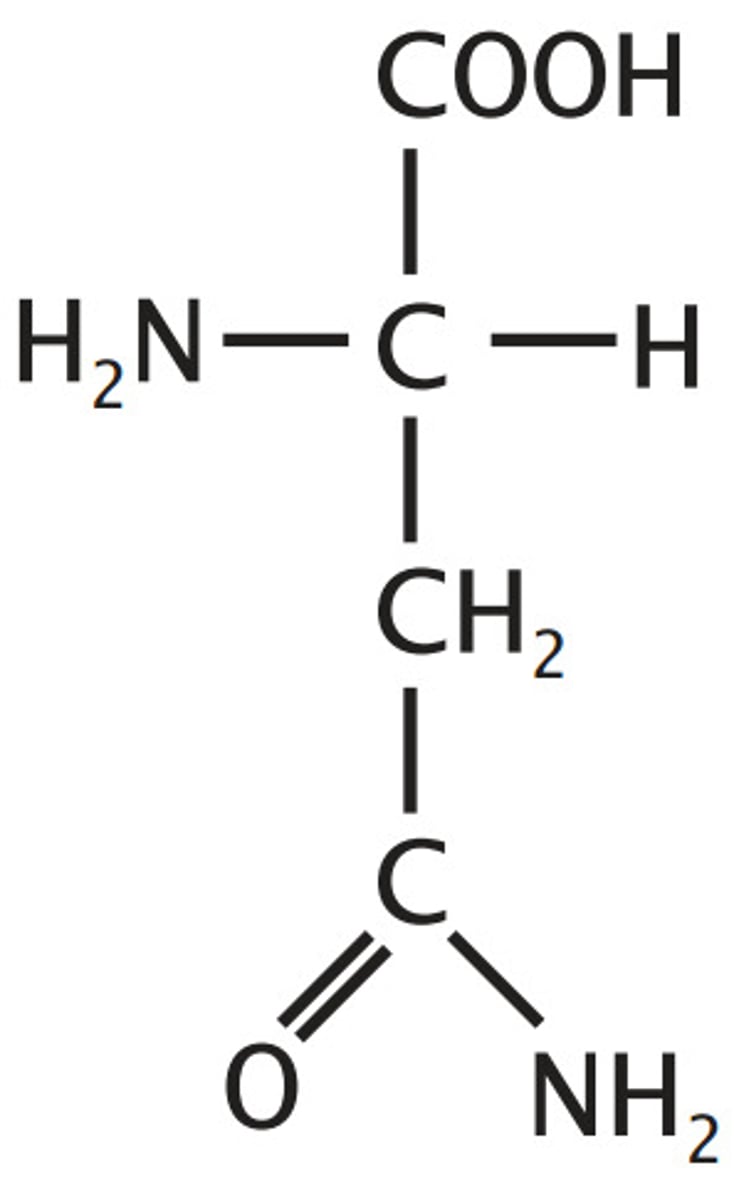
Asparagine
Amide derivatives of acids - loose OH for NH2 to loose charge
amide derivative of aspartate
Glutamine
Amide derivatives of acids - loose OH for NH2 to loose charge
amide derivative of glutamate