lectures 1 biol215
0.0(0)
Card Sorting
1/102
Earn XP
Description and Tags
Last updated 11:04 PM on 9/24/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
103 Terms
1
New cards
CHARECTERSTIC OF LIVING THINSG ACRONYM
ORG HED MR C
2
New cards
characteristics of living things
Living things possess several key characteristics that distinguish them from non-living things. These characteristics include:
1. **Cellular organization**: if you have a cell its organized in a certain way;definite organiztion to living things(bilateral body structure)
2. Reproduction: specifically CELLS can repoduce
3. Growth and development: Living things can grow in size and undergo changes in form and function over their lifespan.
4. Metabolism: Living things have the ability to obtain and use energy through various metabolic processes, such as respiration or photosynthesis.
5. Response to stimuli: Living things can respond to external stimuli in their environment, such as light, temperature, or touch.
6. Homeostasis: Living things maintain a stable internal environment through processes like temperature regulation, pH balance, and water balance.
7. contains one more more cells
8. evolution of population
9. DNA
10. \
These characteristics collectively define what it means to be a living organism.
1. **Cellular organization**: if you have a cell its organized in a certain way;definite organiztion to living things(bilateral body structure)
2. Reproduction: specifically CELLS can repoduce
3. Growth and development: Living things can grow in size and undergo changes in form and function over their lifespan.
4. Metabolism: Living things have the ability to obtain and use energy through various metabolic processes, such as respiration or photosynthesis.
5. Response to stimuli: Living things can respond to external stimuli in their environment, such as light, temperature, or touch.
6. Homeostasis: Living things maintain a stable internal environment through processes like temperature regulation, pH balance, and water balance.
7. contains one more more cells
8. evolution of population
9. DNA
10. \
These characteristics collectively define what it means to be a living organism.
3
New cards
why dont we consider vitus living organisms
Does not have organization,(isnt eczcatly orgaizedjust complex of proetins and nucleic acids)
some don’t have DNA(they have RNA)
, they can only reproduce thorugh host
, no growth and development ,
no reposnse to environment,
no metabolism,
no homeostasis.
Does noy contain ne or more cells
some don’t have DNA(they have RNA)
, they can only reproduce thorugh host
, no growth and development ,
no reposnse to environment,
no metabolism,
no homeostasis.
Does noy contain ne or more cells
4
New cards
virus
complex of proteins and nucleic acid
use ost ti replcate new vrius particles
use ost ti replcate new vrius particles
5
New cards
nucleoprotein complex
A complex formed by the binding of nucleic acids and proteins. It plays a crucial role in various cellular processes, such as DNA replication, transcription, and packaging.
\-can have agenome made of DNA or RNA
\-genome can be double stranded or single stranded
\-can have agenome made of DNA or RNA
\-genome can be double stranded or single stranded
6
New cards
sars-cov2
severe acute repsiroty syndrome-corna virus 2
\-cUAED covid 19
single stranded RNA virus
genome is a single strnande or RNA
\-enters humsn vells by binifng to the ACE2 receptor
\-cUAED covid 19
single stranded RNA virus
genome is a single strnande or RNA
\-enters humsn vells by binifng to the ACE2 receptor
7
New cards
cells
basic unit of life
\-smallest unit witjh the capcity to livee and repoduce\\-eithe rindependnet or par t of muli cellualr organism
\-smallest unit witjh the capcity to livee and repoduce\\-eithe rindependnet or par t of muli cellualr organism
8
New cards
cell theory
the cell is the unit of tructure, physiology and orgaization in living tings
\
thee cell retains adual existence as a distinct entity and a building block in the construction of orgaims
\
all cells rise form preexistin cells
\
thee cell retains adual existence as a distinct entity and a building block in the construction of orgaims
\
all cells rise form preexistin cells
9
New cards
physiology
Definition: The study of how living organisms function and maintain homeostasis at the cellular, tissue, and organ system levels. It explores processes like metabolism, respiration, circulation, and reproduction, providing insights into how our bodies work and adapt to different conditions.
10
New cards
a xylem is an example of a ___
plant cell
11
New cards
a radiolarian is an exampe of a
protist cell
12
New cards
a treoonema is an example of a
bacterial cell
13
New cards
differnce between sars -cov2 and covid 19
Flashcard: SARS-CoV-2 vs. COVID-19
SARS-CoV-2: Virus causing COVID-19. It is a type of coronavirus.
COVID-19: Disease caused by SARS-CoV-2. Symptoms include fever, cough, and difficulty breathing.
Difference: SARS-CoV-2 is the virus, while COVID-19 is the disease it causes.
SARS-CoV-2: Virus causing COVID-19. It is a type of coronavirus.
COVID-19: Disease caused by SARS-CoV-2. Symptoms include fever, cough, and difficulty breathing.
Difference: SARS-CoV-2 is the virus, while COVID-19 is the disease it causes.
14
New cards
how many domains of life are there
3
15
New cards
what is the tree of life
\
\
The Tree of Life is a metaphorical concept that represents the interconnectedness of all living organisms. It illustrates the evolutionary relationships between different species, tracing their common ancestry. It shows how species have branched out over time, forming a complex network of life on Earth.
\
continually being redrsawn
\
continually being redrsawn
16
New cards
early classification systems of tree of life was based on
morpholgical charvetristics
17
New cards
morpholgical charectersrics
Physical features or traits that describe the form and structure of an organism, such as body shape, size, color, and appendages. They play a crucial role in identifying and classifying different species.
18
New cards
scala natue
350 BC
scheme outlined by aristtoles history of animals
all matter organized as decreed by GOD
\-TEMRS STILL USED TODAY:vertebrates and invertabrates
scheme outlined by aristtoles history of animals
all matter organized as decreed by GOD
\-TEMRS STILL USED TODAY:vertebrates and invertabrates
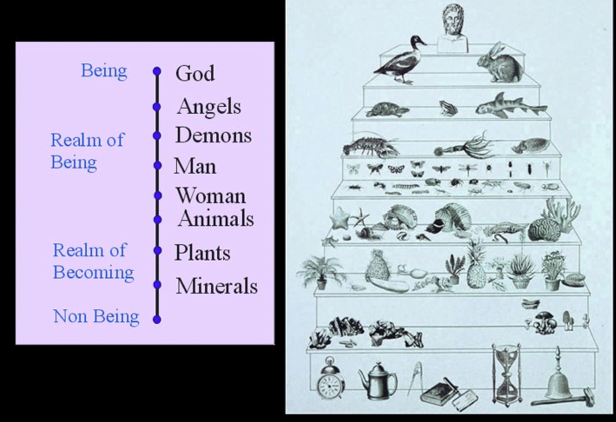
19
New cards
2 kingofms
1700s
pioneered bhy carl lineasy and his binomial taxonomy
ecterythib was either plant or animal
bacteria were comjnsidered planst
pioneered bhy carl lineasy and his binomial taxonomy
ecterythib was either plant or animal
bacteria were comjnsidered planst
20
New cards
5 kingdom
1960s
monera(prokaryotes)
prosita
plante
fungi
animilia
monera(prokaryotes)
prosita
plante
fungi
animilia
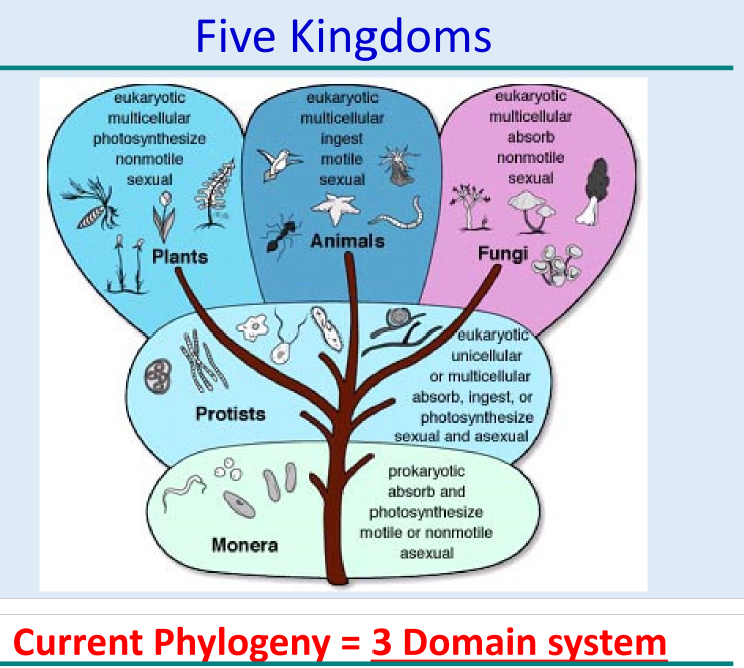
21
New cards
current phylogeny system
3 domain system
\
bacteria=prokaryotes
archaea=prokaryoes(many extremophiles)
Eukarya=eukaryotes
\-protist , plantsk, fungi, anmals
\
bacteria=prokaryotes
archaea=prokaryoes(many extremophiles)
Eukarya=eukaryotes
\-protist , plantsk, fungi, anmals
22
New cards
what did they use to establish current tree of life and why
USED RRNA gene sequences to establish relations between iving things
Whyd they do this?
\-they are highly CONSERVED among all organisms
\-plus all living things contain it(all living things have ribosomes so hey all have that gene)
23
New cards
who pioneered currnt tree of life and when was it recogized
carl woses and george fox peionerered it in the late 1970s but it was frmally recohnized in 19902
24
New cards
why is it important to use rRNA
**-all cells require ribsomal RNA(RRNA)**
**-highly conserved:**changes slowly with thime;this mean important sequences that do not evolve rapidly;any small change reflects an evolutionary steps\\
\-can be used to establish evolutionary relationships btween all secies
\-conserved regions enable easy in virto replication
\-ie:we kow hese sequences therefore we can design PCR primers
25
New cards
analysus of rRNA genes revealed
two seperate groups of prokryotes
suggested that eukaryotes and archeas are more closely related o each other than to bacteria
allowed better understanding of how protist should be organized phylogenetically(revealed how badit was to classify all orotis together,,some arent even related)
\
suggested that eukaryotes and archeas are more closely related o each other than to bacteria
allowed better understanding of how protist should be organized phylogenetically(revealed how badit was to classify all orotis together,,some arent even related)
\
26
New cards
what are the 2 seperate groups of prokaryotes
bacteria and archean domains
27
New cards
simialrities of eukaryotes and archaea
\
\
Despite this visual similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely-related to those of eukaryotes, notably the enzymes involved in transcription and translation. Archaea exhibit a great variety of chemical reactions in their metabolism and use many sources of energy.
28
New cards
protist
diverse group of eukaryotic organisms
A diverse group of eukaryotic microorganisms that are not plants, animals, or fungi. They can be unicellular or multicellular. Protists play important roles in various ecosystems as producers, consumers, and decomposers. They can be found in freshwater, marine environments, and even in soil. Some examples include amoebas, algae, and paramecia.
they are NOT all single cells
A diverse group of eukaryotic microorganisms that are not plants, animals, or fungi. They can be unicellular or multicellular. Protists play important roles in various ecosystems as producers, consumers, and decomposers. They can be found in freshwater, marine environments, and even in soil. Some examples include amoebas, algae, and paramecia.
they are NOT all single cells
29
New cards
unicellular protusts example
dinoflagellates
30
New cards
dinoflagellates
Flashcard: Dinoflagellates
* Microscopic marine organisms
* Possess two flagella for movement
* Photosynthetic or heterotrophic
* Some produce bioluminescence
* Important in marine food chains
* Some species cause harmful algal blooms
* -red tide
* Microscopic marine organisms
* Possess two flagella for movement
* Photosynthetic or heterotrophic
* Some produce bioluminescence
* Important in marine food chains
* Some species cause harmful algal blooms
* -red tide
31
New cards
multicellar protist example
algae
32
New cards
Describe the 3 domains of life
1. Bacteria: Single-celled organisms with no nucleus, found everywhere on Earth.
2. Archaea: Also single-celled, but can survive extreme conditions like hot springs or deep oceans.
3. Eukarya: Complex organisms with a nucleus, including plants, animals, fungi, and protists.
33
New cards
What is a protist? Why is it no longer correct to classify all protists together into one phylogenetic group
\
\
A protist is a diverse group of eukaryotic microorganisms that do not fit into the categories of plants, animals, or fungi. It is no longer correct to classify all protists together into one phylogenetic group because advances in molecular biology have revealed that protists are not a monophyletic group. They are now recognized as belonging to multiple evolutionary lineages, each with distinct characteristics and evolutionary histories.
34
New cards
Explain the four steps leading to the evolution of the first cells.
1) abiotic synthesis of simple organic compounds(ex nitorgenous base or amino acids)
2)abiotic suntheiss of organic macromolecular polumers(ex nuclc acids)
3) evolution of an informatio sargae molcule caabl of replication
4)formatio of membrane surronding the information stargae molecule
2)abiotic suntheiss of organic macromolecular polumers(ex nuclc acids)
3) evolution of an informatio sargae molcule caabl of replication
4)formatio of membrane surronding the information stargae molecule
35
New cards
Explain the four steps leading to the evolution of the first cells. in depth
1. Abiotic Synthesis of Simple Organic Compounds: This step involves the formation of basic organic molecules, such as amino acids (building blocks of proteins) and nitrogenous bases (components of nucleic acids like DNA and RNA), from non-living or inorganic sources. This process might have occurred through various natural processes like lightning, volcanic activity, or reactions in the early Earth's atmosphere and oceans. The presence of these simple organic molecules was a crucial prerequisite for the formation of more complex structures.
2. Abiotic Synthesis of Organic Macromolecular Polymers: In this step, the simple organic compounds from the previous step would have reacted and combined to form larger and more complex molecules called macromolecules. For example, amino acids could have linked together to form proteins, while nucleotides (including the nitrogenous bases) could have joined to form nucleic acids like DNA and RNA. These macromolecules are the building blocks of life and carry out essential functions within cells.
3. Evolution of an Information Storage Molecule Capable of Replication: This step refers to the emergence of a molecule capable of storing genetic information and replicating itself. The most well-known examples of such molecules are DNA and RNA. These molecules can store genetic instructions that guide the development, functioning, and reproduction of organisms. The ability to replicate is crucial for passing on this genetic information to the next generation. The emergence of a self-replicating molecule marked a significant leap toward the establishment of a primitive form of life.
4. Formation of a Membrane Surrounding the Information Storage Molecule: With self-replicating molecules present, the next step was the development of a boundary or membrane around these molecules. A membrane would have helped to separate the internal processes of these early "cells" from their external environment. This separation is essential for maintaining distinct chemical environments and allowing for controlled interactions with the surroundings. Membranes could have formed spontaneously from lipids or other molecules, creating a protective barrier for the evolving cellular structures.
36
New cards
miller - urey exeriment
in 1953 stnley miller and harold urey tested the hyptohesis that energy from lughtining could have powered producion of siple orgnic compounds from reuced atoshperic gaes
\
after a week of continous exposure of gasses to electrical dscharge miller checked the flask
\
he deteced two simple amino acids which were alanine and glycine in the flask
\
this sugestd that some organic compounds could be produced under abiotic conditions
\
after a week of continous exposure of gasses to electrical dscharge miller checked the flask
\
he deteced two simple amino acids which were alanine and glycine in the flask
\
this sugestd that some organic compounds could be produced under abiotic conditions
37
New cards
earths atmospher was largely
hydorgen
methae
ammonia
water vapor
\
it was a very hostile eniorment named the hadeon era
methae
ammonia
water vapor
\
it was a very hostile eniorment named the hadeon era
38
New cards
earths curent aytmosphere
78% nitorgen
21% oxygen
0\.9% argon
0\.1% other
21% oxygen
0\.9% argon
0\.1% other
39
New cards
prokaryotes vs eukaryotes
Prokaryotes: Simple cells lacking a nucleus and membrane-bound organelles. Examples include bacteria and archaea.
Eukaryotes: Complex cells with a nucleus and membrane-bound organelles. Examples include plants, animals, fungi, and protists.
Eukaryotes: Complex cells with a nucleus and membrane-bound organelles. Examples include plants, animals, fungi, and protists.
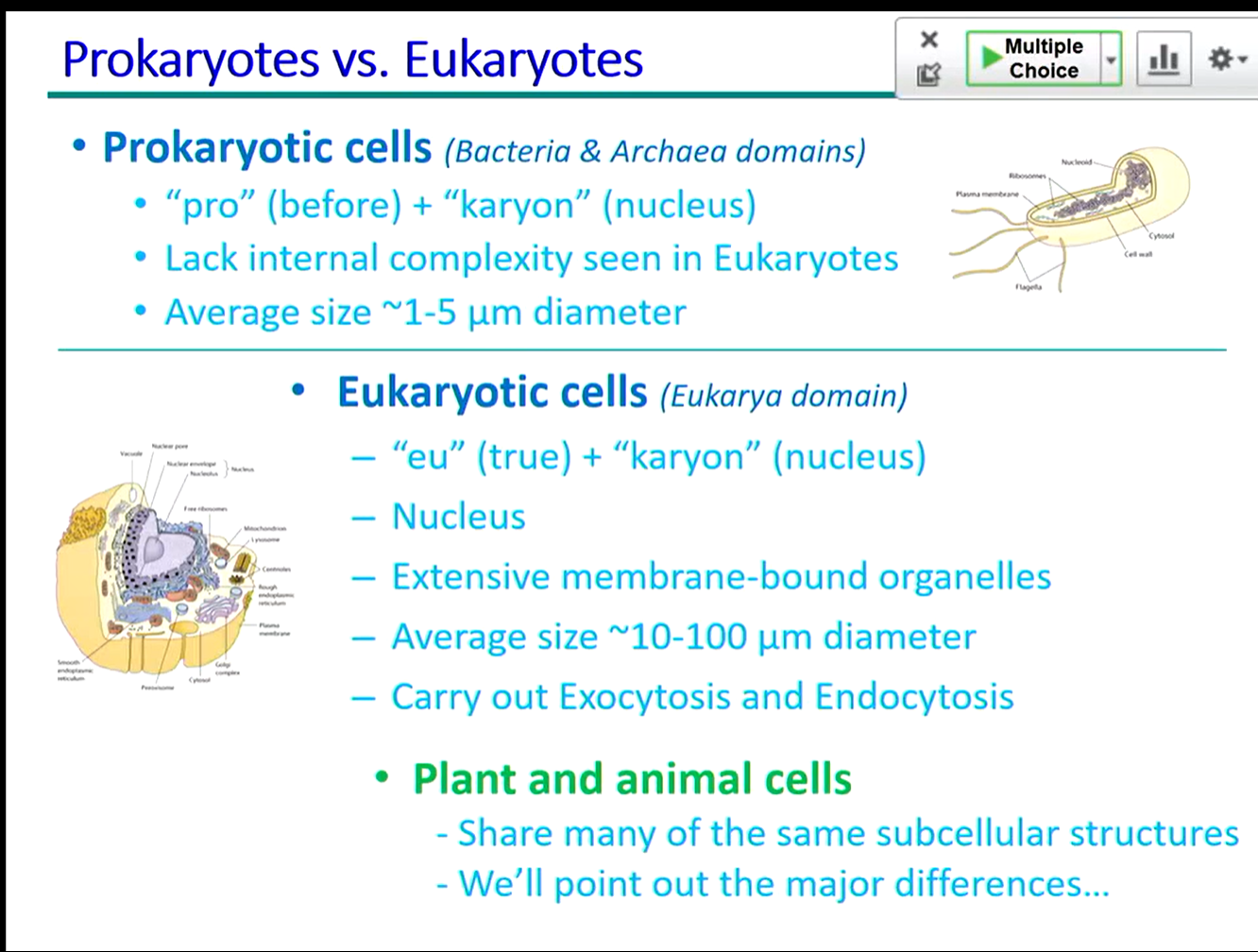
40
New cards
prokaryotes iclude which oranisms:bacteria, archaea, protist
bacyeria and archaea
41
New cards
prokaryotes vs eukaryotes
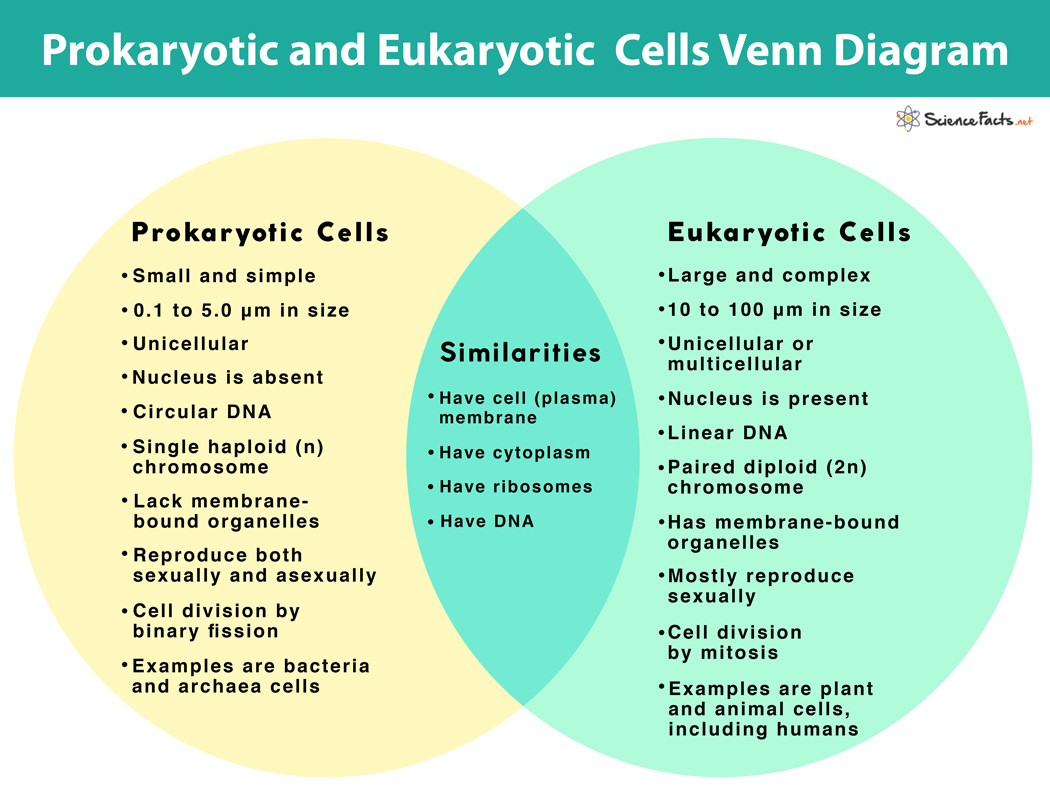
42
New cards
avergae size of prokarytoic cell
1-5 um diamete
43
New cards
average size of eukaryotic cell
10-100um
44
New cards
giga
G, 10^9
45
New cards
number exponents meaning
10^3=3 a34ow
46
New cards
mega
M, 10^6
47
New cards
kilo
k, 10^3
48
New cards
deci
d, 10^-1
\
\
49
New cards
centi
c, 10^-2
50
New cards
mili
10^-3
51
New cards
micro
u, 10^-6
52
New cards
nano
n, 10^-9
53
New cards
pico
p, 10^-12
54
New cards
femto
f, 10^-15
55
New cards
what unit lengt would beused to measure the lenght of a bcterial cll
micrometers; used for expressing size of cells annd larger organelle
56
New cards
nanometer (nm) or angstrom(A) the a has a degrree symbol on top
units used to express size of molecules and smaller subcellular structures
57
New cards
angstrom
0\.1 nm or 10^-10m
\
commomly used to express dimension fo molecules
\
commomly used to express dimension fo molecules
58
New cards
dalton
atmic mas unit commonly used to express size of proteins
59
New cards
go to canvas and do units worksheet
!
60
New cards
saccharomyces cerevisiae
Type of yeast used in baking and brewing, known as baker's yeast or brewer's yeast. It ferments sugars to produce carbon dioxide and alcohol, helping dough to rise and giving beer its alcohol content.
61
New cards
cell divison pattern in saccharomyces cerevisiae
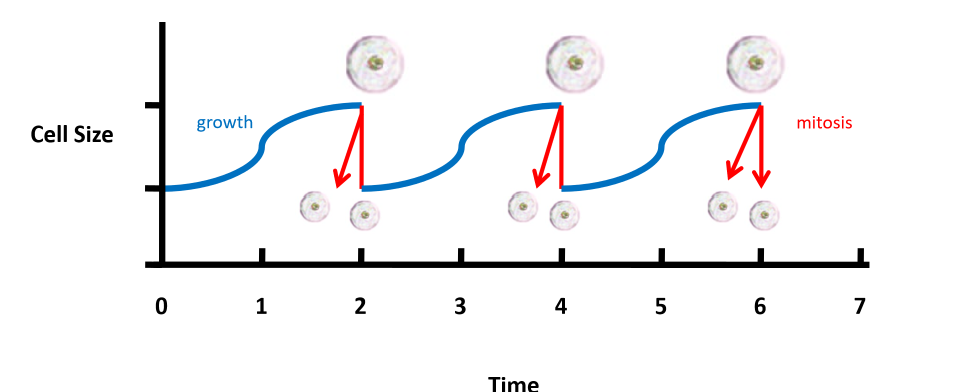
62
New cards
R is ana brrievation for
the ret of the molecule
could be any type of organic molecule with any number of hydrocarbons
could be any type of organic molecule with any number of hydrocarbons
63
New cards
hydrocarbon
carbon with hydrogen attached
64
New cards
functional groups
specific group of aroms added to organic molecules, lend secific chemical charceterstics to molecules which group occurs
65
New cards
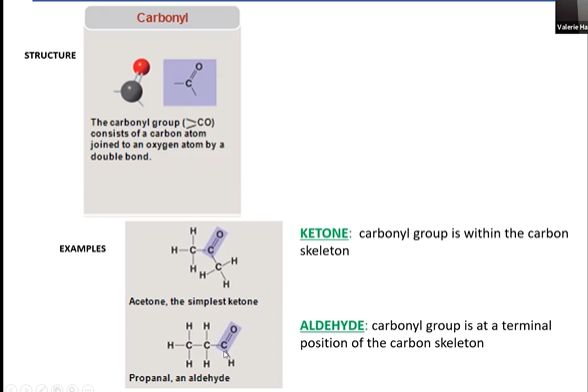
carbonyl
\
\
\
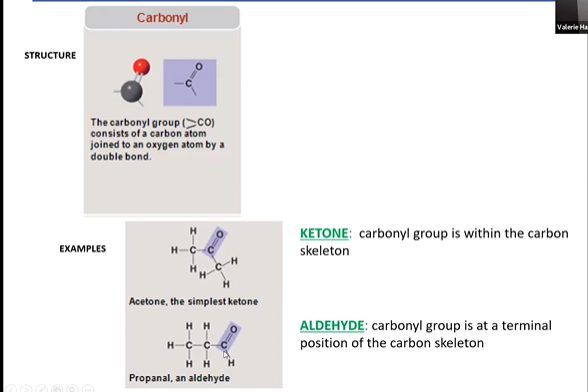
66
New cards
aldehyde
An aldehyde is a type of organic compound that contains a carbonyl group (C=O) bonded to a carbon atom at the end of a carbon chain. Aldehydes are characterized by the presence of a hydrogen atom or alkyl group attached to the carbonyl carbon. They are important in various chemical reactions and are commonly found in substances like essential oils and sugars.
\
\
67
New cards
ketone
ketone is a type of organic compound containing a carbonyl group (C=O), where the carbon atom of the carbonyl group is bonded to two other carbon atoms. Ketones are characterized by having the carbonyl group located within the carbon chain. Acetone (CH₃COCH₃) is a common example of a ketone.
68
New cards
structural isomer
Definition: Structural isomers are a type of isomerism in which two or more compounds have the same molecular formula but different arrangements of atoms. This results in distinct chemical structures and often leads to differences in physical and chemical properties. Example: 1. Butane and Isobutane: - Butane: CH₃CH₂CH₂CH₃ - Isobutane: (CH₃)₃CH These two compounds have the same molecular formula (C₄H₁₀) but different arrangements of carbon atoms, resulting in different physical properties and behaviors. 2. Ethanol and Dimethyl Ether: - Ethanol: CH₃CH₂OH - Dimethyl Ether: CH₃OCH₃ These compounds share the same molecular formula (C₂H₆O) but differ in the connectivity of atoms, leading to distinct chemical properties. Structural isomers illustrate the importance of molecular arrangement in determining a compound's characteristics and reactivity.
\
\
69
New cards
aldehydes and ketons are commonly found in
monossacharides(carbs)
70
New cards
monosaccharide
Definition: A monosaccharide is the simplest form of carbohydrate, often referred to as a single sugar molecule. It consists of a single polyhydroxy aldehyde or ketone unit. Monosaccharides are the building blocks of more complex carbohydrates and serve as a primary source of energy for living organisms. Examples include glucose, fructose, and galactose.
\
\
71
New cards
what di you call smth that contains an aldehyde
aldose sugar
72
New cards
what di you call smth that contains an ketone
ketose sugar
73
New cards
types of carbonyl
acetyl, adehylyde, ketoe, carboxyl/carboxylic acid
74
New cards
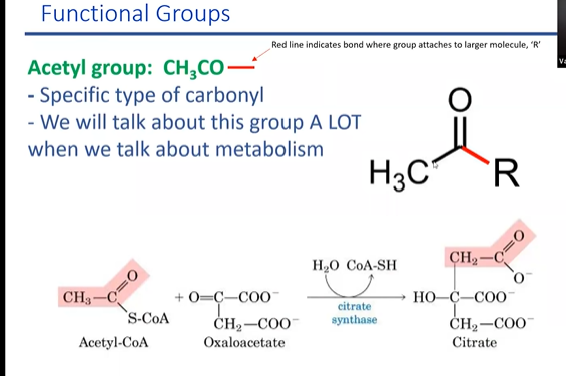
acetal group
75
New cards
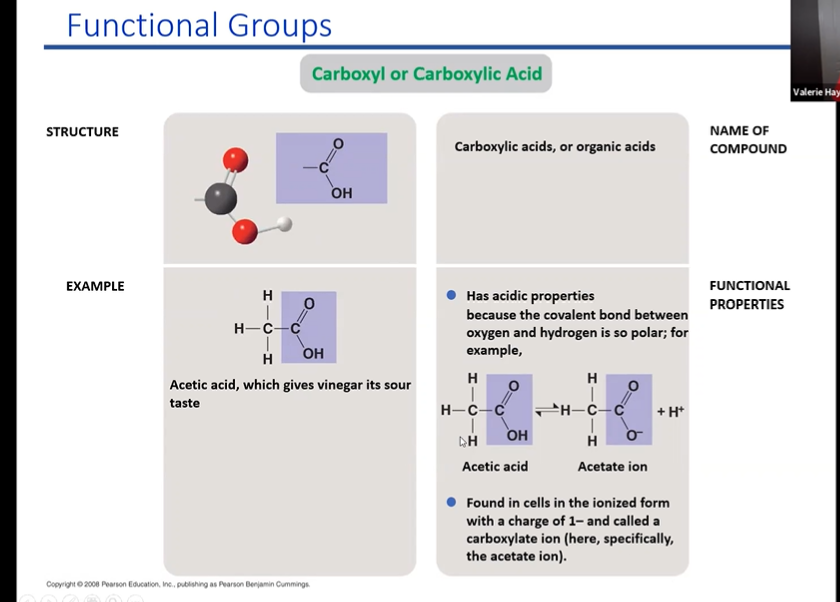
carboxyl/carboxylic acid
76
New cards
when an acid is put into a solution
it gives off a proton and makes solution more acidic;loses hydrogen on OH
77
New cards
carboxyl vs carbocylic acid
The carboxyl group contains the C=O. of the carbonyl group, with the carbon atom also being bonded to a hydroxyl (−OH) group. A carboxylic acid is an organic compound that contains the carboxyl functional group. The general formula for a carboxylic acid can be abbreviated as R−COOH
78
New cards
hydroxyl
when put into a solution it is polar but does not become charged
\
the o and h are covalently bonded
\
polar b/c oxygen has greater electernegatuvy
\
this mean oxygen likes electrons more so it has a partial negative and hydro has parila psoitive
\
the o and h are covalently bonded
\
polar b/c oxygen has greater electernegatuvy
\
this mean oxygen likes electrons more so it has a partial negative and hydro has parila psoitive
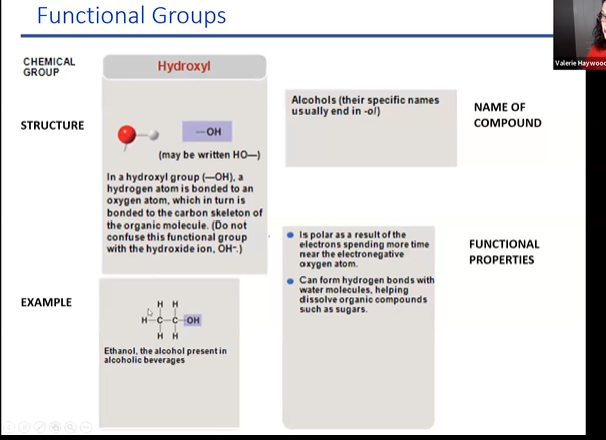
79
New cards
what does this mena:when put into a solution it is polar but does not become chargd
The statement "when a hydroxyl is put in a solution it is polar but does not become charged" refers to the behavior of a hydroxyl group (-OH) when it is dissolved in a solvent, typically water. Let's break down the key concepts: 1. \*\*Polar:\*\* A polar molecule is one that has an uneven distribution of electron density, resulting in partial positive and partial negative charges within the molecule. This polarity arises when atoms with different electronegativities are bonded together, causing electron clouds to be more concentrated around one atom than the other. In the case of a hydroxyl group (-OH), the oxygen atom is more electronegative than the hydrogen atom, leading to a partial negative charge on the oxygen and a partial positive charge on the hydrogen. 2. \*\*Charged:\*\* A charged molecule, also known as an ion, has either gained or lost electrons, resulting in a net positive or negative charge. For example, a positively charged ion is called a cation, and a negatively charged ion is called an anion. In the context of the statement, "when a hydroxyl is put in a solution it is polar but does not become charged," it means that when a hydroxyl group is dissolved in a solution (usually water), its oxygen atom and hydrogen atom maintain their partial charges due to the polarity of the hydroxyl group. However, this does not result in the hydroxyl group gaining or losing electrons to become fully charged ions. Instead, the partial charges on the oxygen and hydrogen atoms interact with the polar solvent molecules (like water), leading to interactions like hydrogen bonding, which are important for various chemical and biological processes. In summary, the hydroxyl group's polarity is retained when it is dissolved in a solution, contributing to its interactions with other molecules, but it does not gain or lose electrons to become fully charged like ions do.
80
New cards
protonated
Meaning: Protonated refers to a molecule, ion, or atom that has gained or accepted a proton (H⁺) during a chemical reaction. This process typically results in an increase in positive charge due to the addition of a positively charged proton. Protonation often occurs in acidic environments where there are excess protons
\
\
81
New cards
charge and protonaion carboxylic acd ad carboxylate
fucntions as an acid when you put it into soltuion. when acid put into solution it gives off a proton making soltuion more acidic. this causes them to lose a hydrogen on the OH and therefore becomes negatly charged in the soltuion
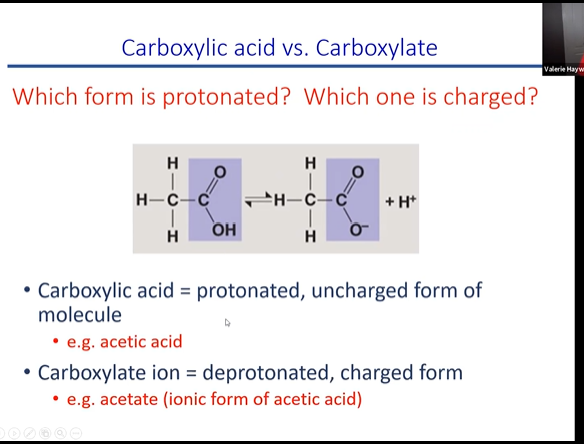
82
New cards
why does a carboxuylic acid funcional group behave diffrently in soltuion(aq) compared to hydroxyl
83
New cards
amino
when you put amine gorup into a soltuion its gonna accept a hydeogen and become prositibly charged
\
accepts h+ from nh3
\
accepts h+ from nh3
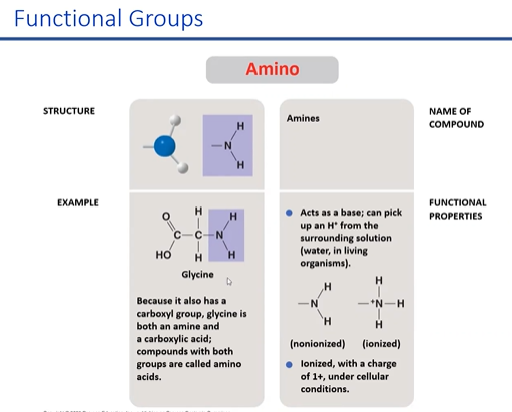
84
New cards
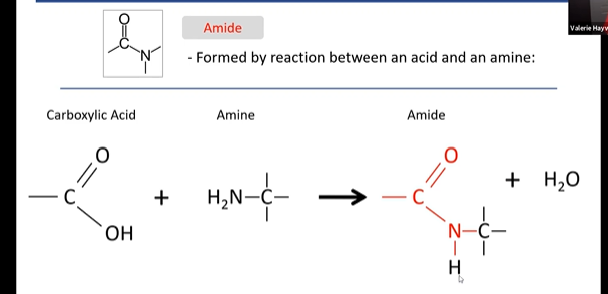
amide
does not take on a charge, remians polar but neutral unlike AMINE, AMIDESS are polar unchaged in solution
85
New cards
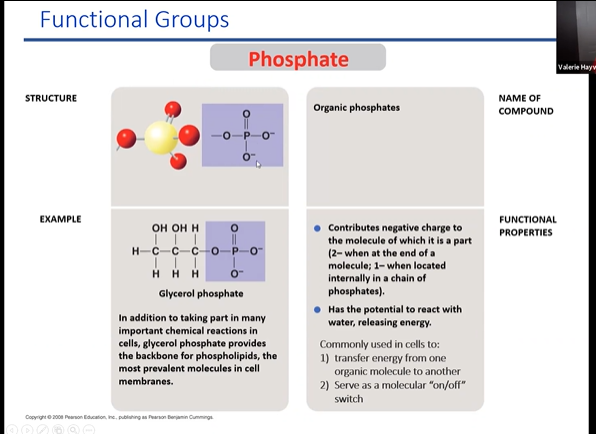
phosphate
regualrory groups to chnage confirmation protein. deactivates and activates molecule
releases h+
releases h+
86
New cards
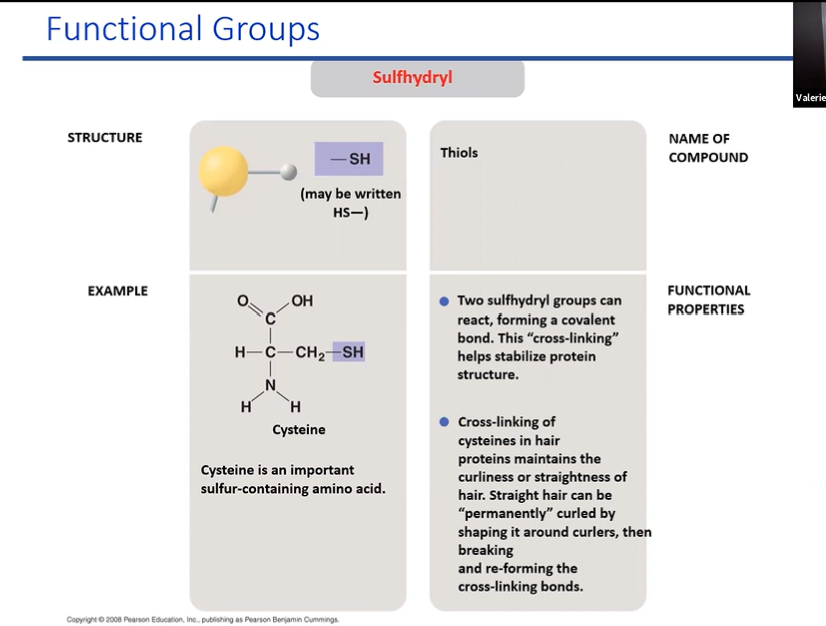
sulfhydryl
when atatched to copund we call them thions
87
New cards
lisyt of funcional groups
88
New cards
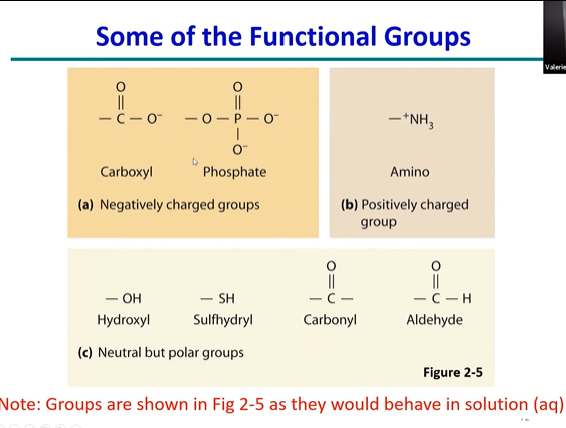
draw fucntional groups in a state where they are in soltuions
(add charges)
(add charges)
89
New cards
amino acid structure
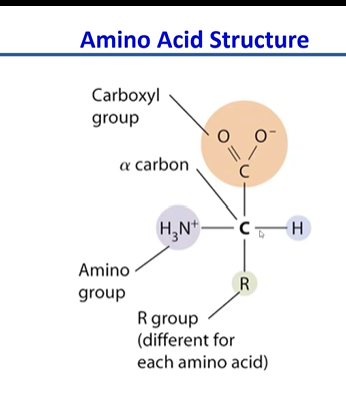
90
New cards
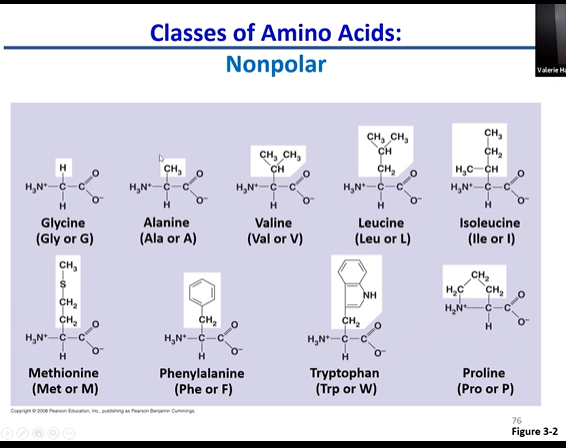
nonpolar amino acids
only focus on r group
mostly hydrocarbon/non polar
mostly hydrocarbon/non polar
91
New cards
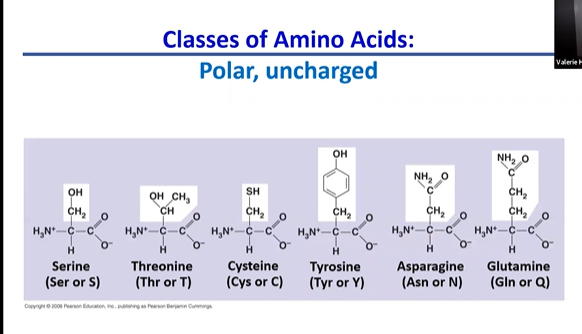
polar uncharged
92
New cards
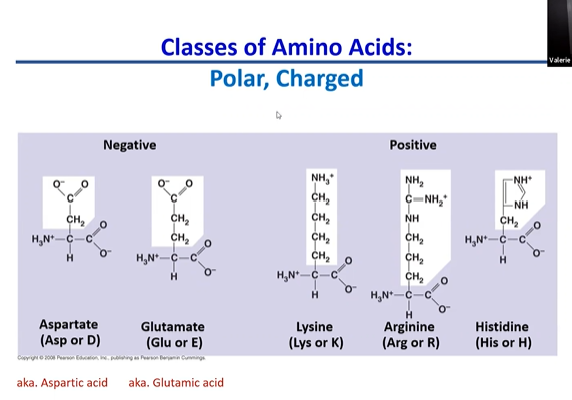
polar charged
93
New cards
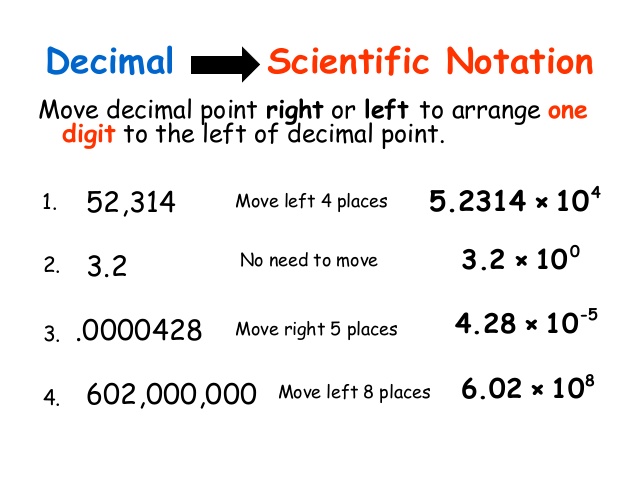
hwo to convert between standard notation and scientific notation.
94
New cards
Robert Hooke
Curator of Instruments for the Royal Society of London; early microscopist. Contributions: In 1665, used a microscope to examine cork and observed small compartments he called "cells.
95
New cards
Cells (Hooke's Observation)
Robert Hooke observed and sketched small, boxlike compartments in cork under a microscope. He named them "cells," inspired by their resemblance to a honeycomb.
96
New cards
Antonie van Leeuwenhoek
Role: Dutch textile merchant, microscopist. Contributions: Created lenses with higher magnification (300x) than Hooke's microscope. Observed living cells such as blood cells, sperm cells, bacteria, and single-celled organisms in pond water.
97
New cards
Microscope Resolution
The ability of a microscope to reveal fine structural details. Significance: Limited microscope resolution hindered understanding of cell structure. Even van Leeuwenhoek's improved lenses had their limits.
98
New cards
Seventeenth-Century Biology
Descriptive in nature, focused on observation rather than explaining biological structures. Impact: Hindered the deeper understanding of the architectural details of cells and other biological components
99
New cards
Compound Microscope
Microscope with an eyepiece lens and an objective lens that work together to magnify images. Advantages: Higher magnification and better resolution; enabled clearer visualization of structures as small as 1 micrometer.
100
New cards
Developments in 1830s
Achievements: Improved lens quality and compound microscope design. Outcome: Enhanced magnification and resolution, allowing clear visualization of structures as small as 1 micrometer.