Chapter 10 Structure and Synthesis of Alcohols
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Alcohols
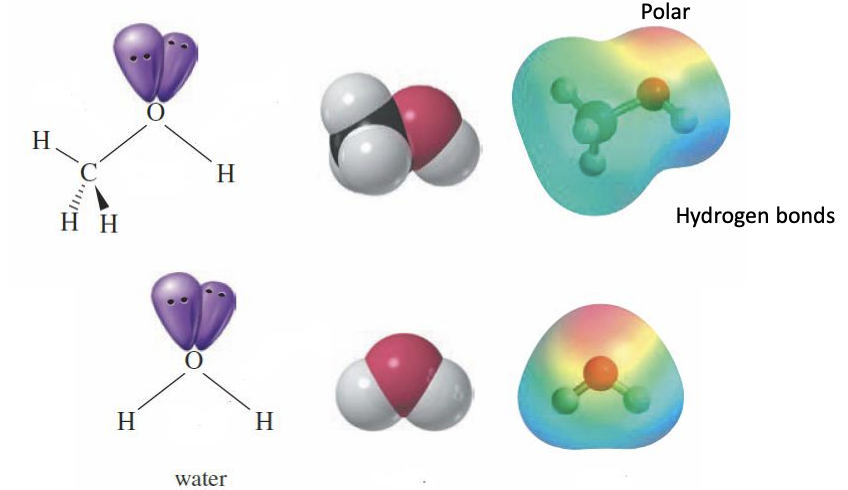
They are polar with hydrogen bonds (with Hydroxy OH group)
Primary Alcohol
Structure with Oh and 1 r group
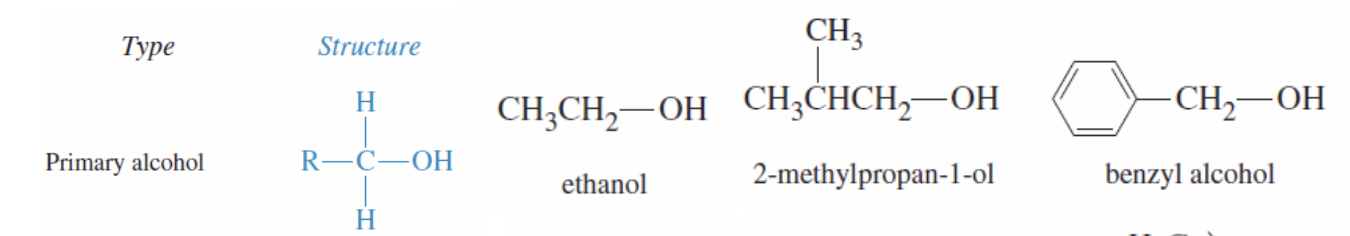
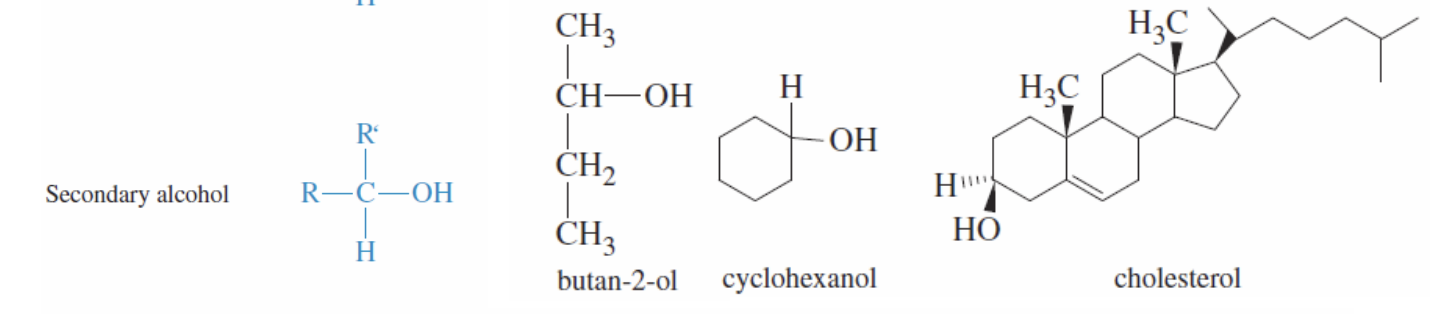
Secondary Alcohol
Structure with Oh and 2 r groups
Tertiary Alcohol
Structure wth OH and 3 R groups

Phenol
Cyclohexene with OH attached (EXTREMLEY ACIDIC)
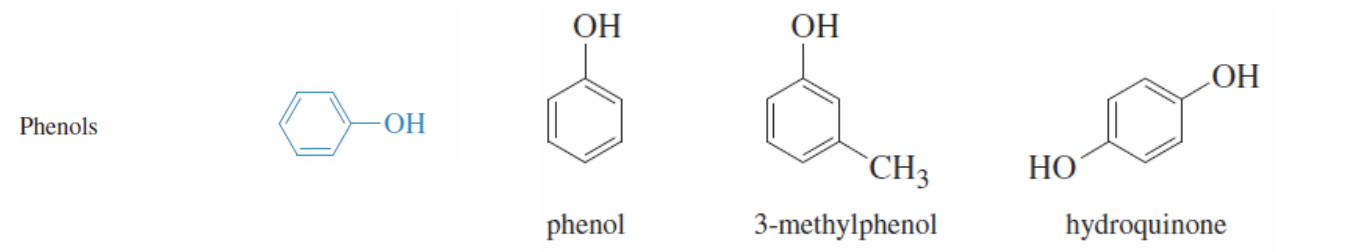
IUPAC nomenclature of Alcohols
Find longest carbon chain with the OH group
number the chain starting form the end closest to the Oh group group
ending name with ol not e
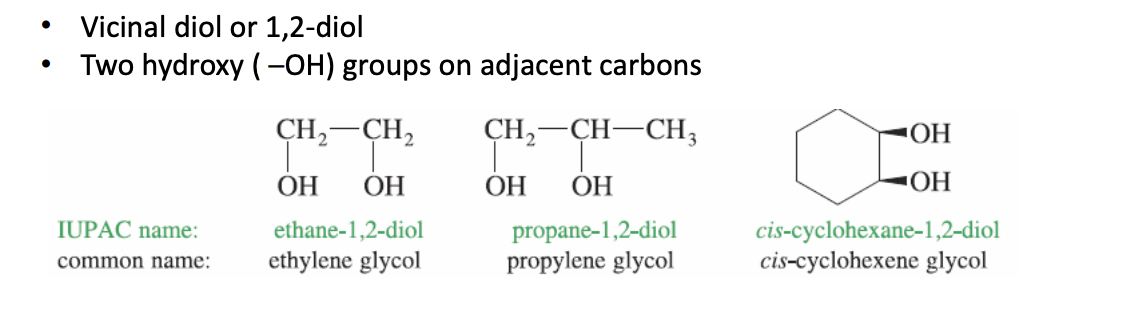
Glycols
Cyclohexane with 2 adjacent hydroxy -oh groups
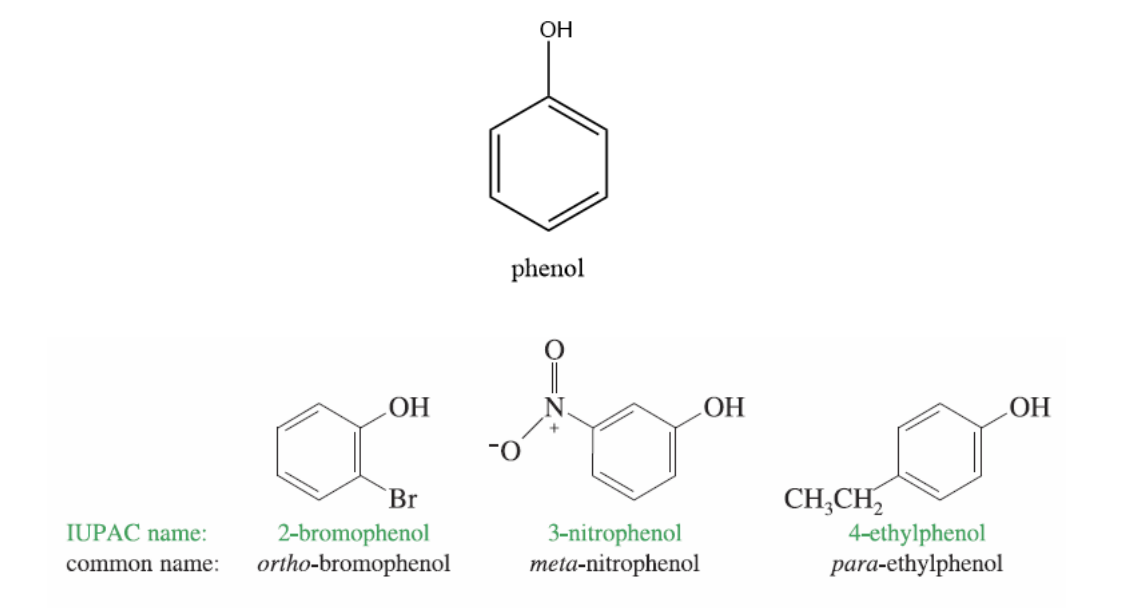
Naming Phenols
OH group is assumed to be on carbon 1
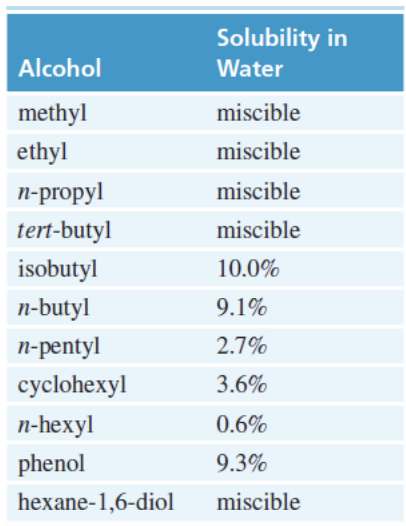
Alcohol Physical Properties
Have High boiling points = due to the hydrogen bonding
alkyl group size increases—> alcohol solubility decreases

Acidity of Alcohols
large pKa of 15.5-18 = weak acid
alkyl group increases = acidity decreases
Added halogens = increases acidity
Alchol Acidity by Classification: Primary alcohol (1°) > Secondary (2°) > Tertiary (3°)
Sodium Alkoxides formation
A primary alcohol reacts with NA to form an IRREVERSIBLE primary alkoxide
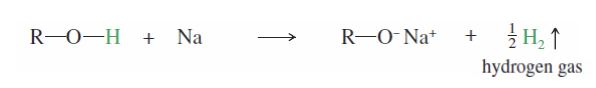
alkoxide: R-OH
Potassium Alkoxides
Secondary or Tertiary alcohol reacts with P to form an IRREVERSIBLE secondary or tertiary alkoxide
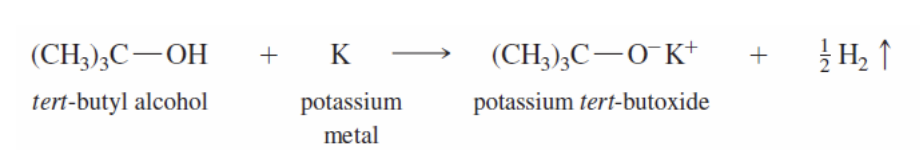
alkoxide: R-OH
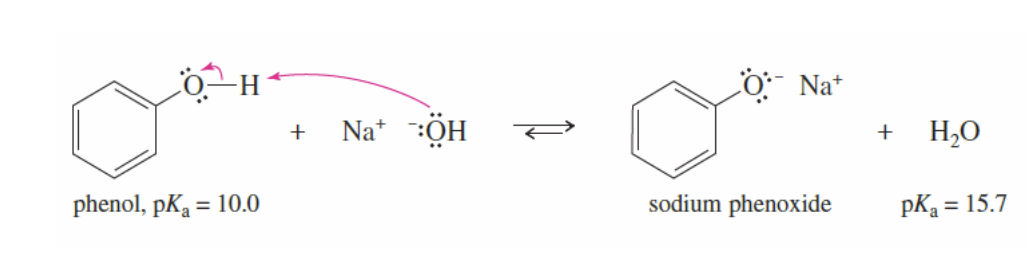
Phenoxide Ion Formation
Phenol reacts with a strong base + -OH (ex, Na+ -OH) to form a phenoxide ion
SN2 Nucleophilic Substitution on an Alkyl Halide
Alkly Haldie + Nuc (OH) —> PrimaryAlcohol + x-
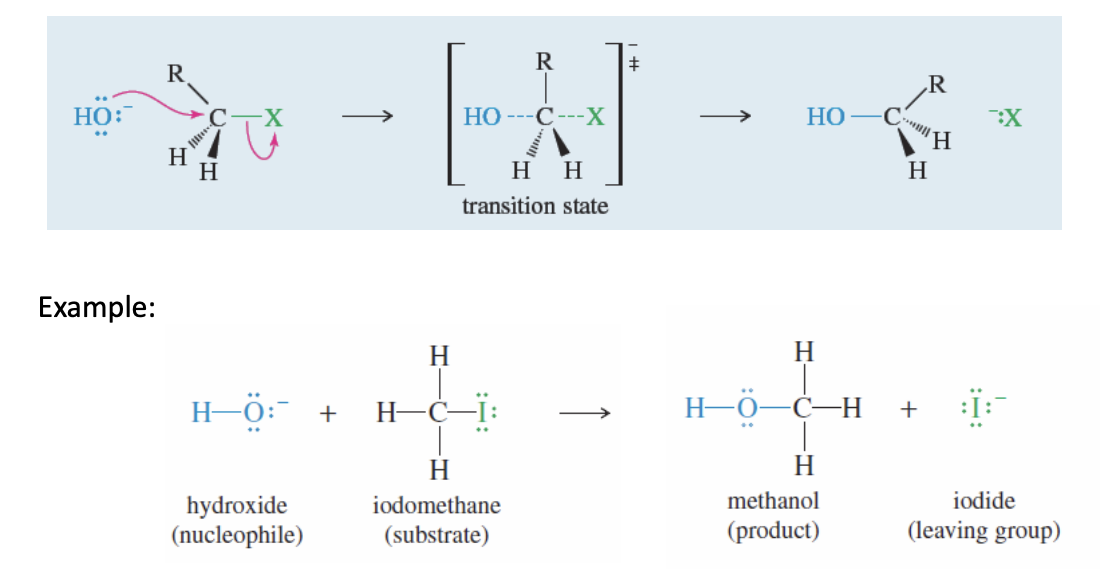
It is the same process of SN2 substitution but only using the specific substrate of an alkyl halide
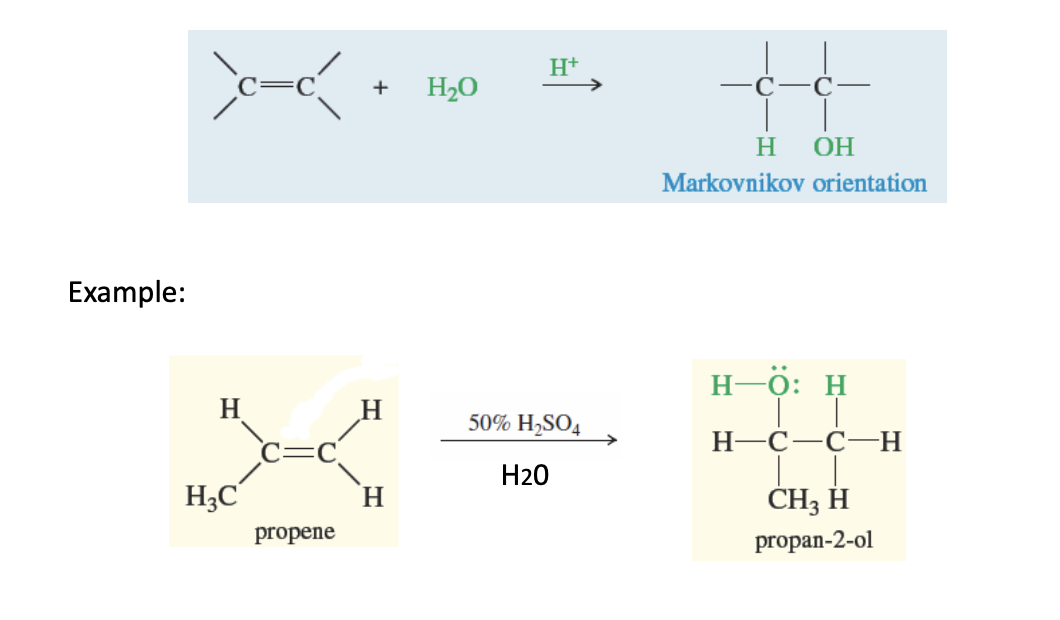
Acid-Catalyzed Hydration of an Alkene (OR JUST ALKENE HYDRATION)
alkene + H20 —> H2so4, h202, or h3Po4
the acids (H+) are the H2SO4, H2O2 and H3PO4
markovinkov
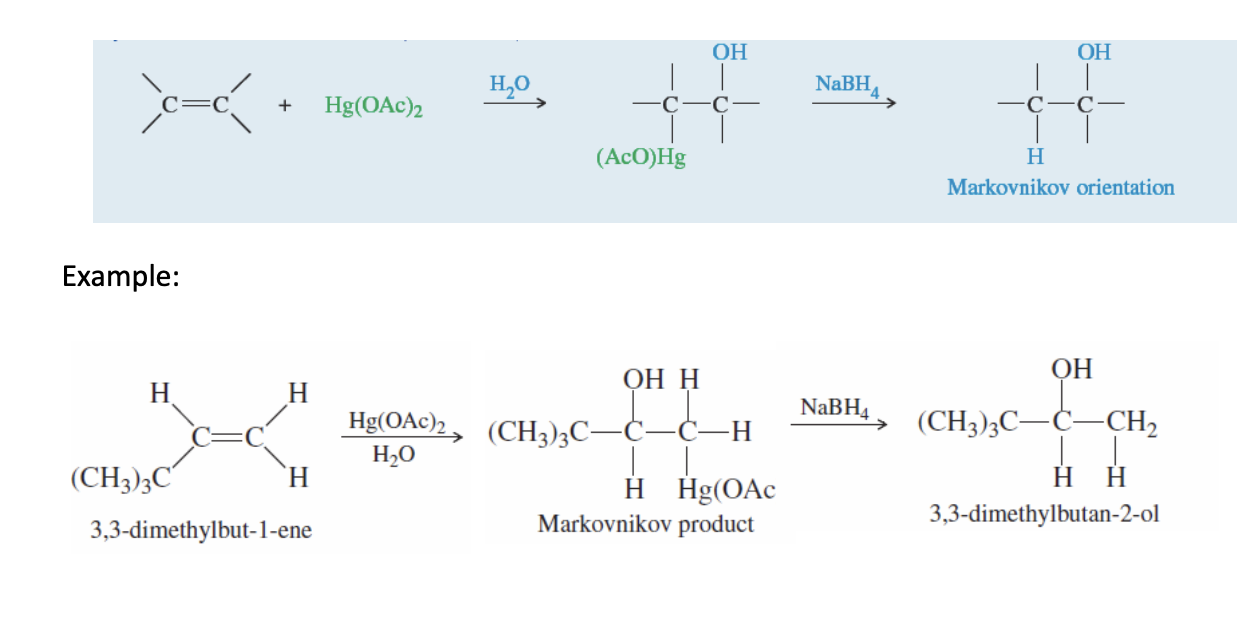
Oxymercuration-Demercuration
Alkene + HgOAC2 —> H2O, H2O —> NABH4
markovinkov
Hydroboration-Oxidation (Or just Hydroboration mechanism)
Alkene + BH3.THF —> H20 (or H2O2, NAOH, -OH dilute BH2 to OH)
syn addition and antimarkovinkov
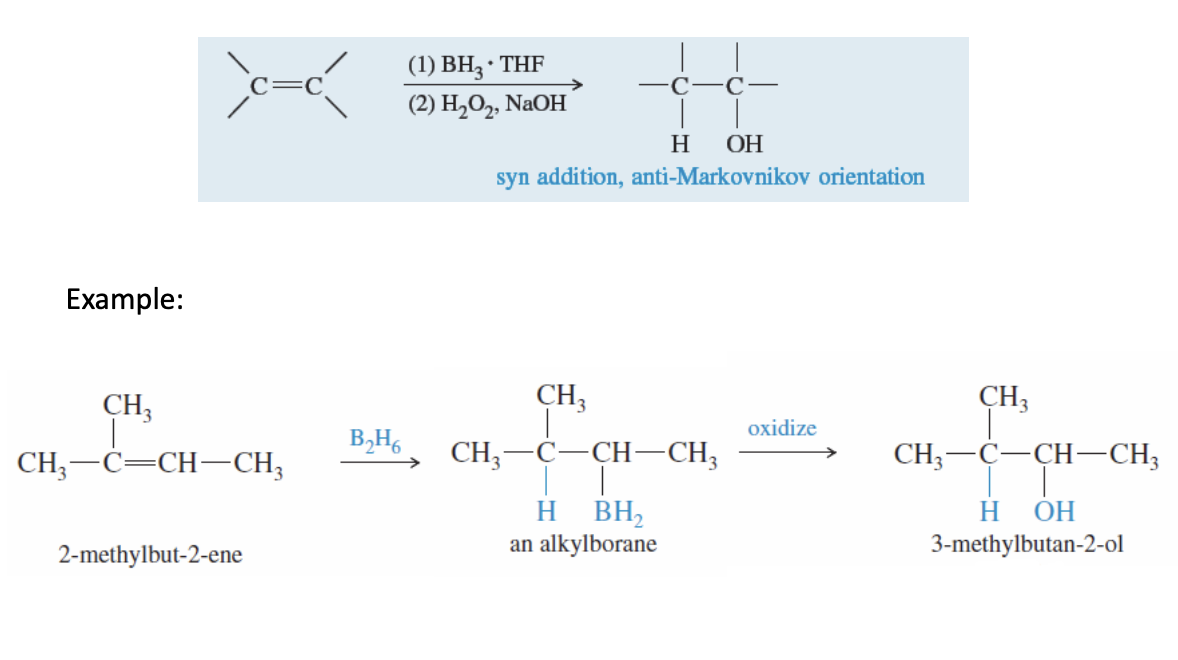
B2H6 can be used instead of BH3.THF
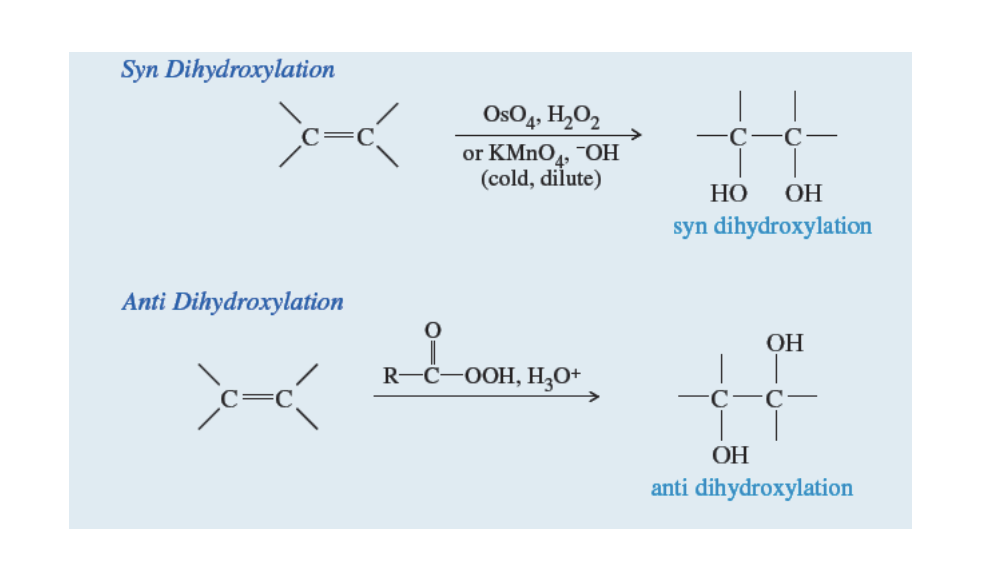
When mechanisms form 1,2-diols formed from alkenes
Two types of 1,2-diols: Antio diol and Syn diol
Antio Diol: Formed through epoxidation
alkene + Peroxyacid —> H+ —> H2O, H2O
Syn Diol: Formed through Synhydroxylation
Alkene +OsO4 —> H2O2 or KMnO4 or -OH
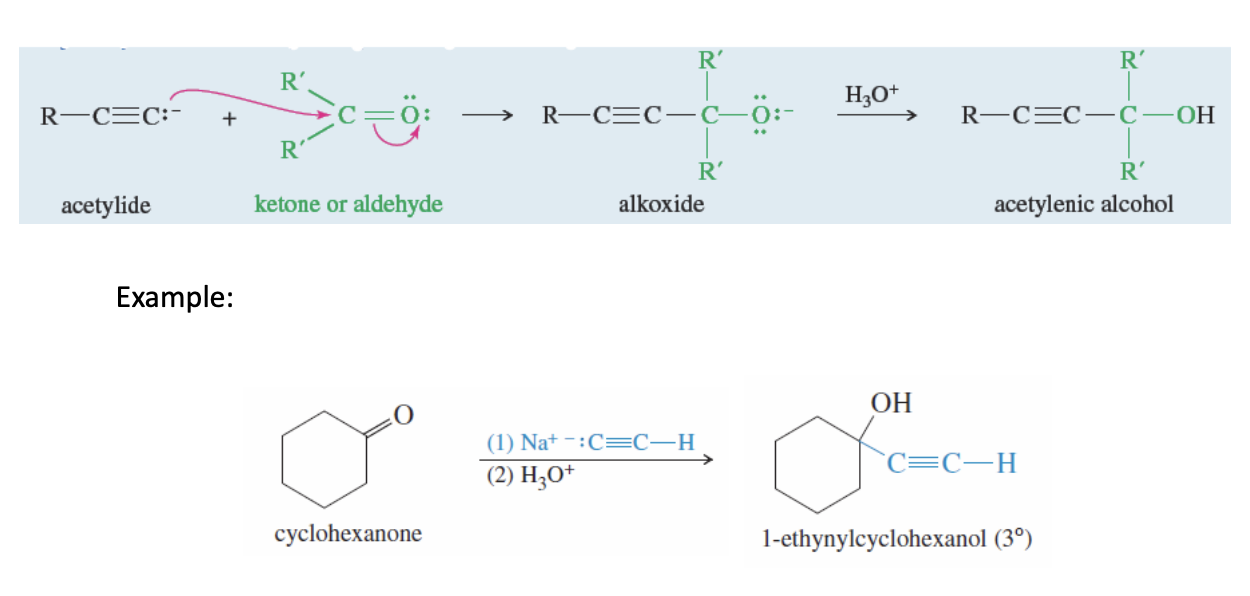
Acetylide Addition to Carbonyls
Acetylide + Ketone (or an aldehyde —> H3O+ —> forms acetylenic alcohol
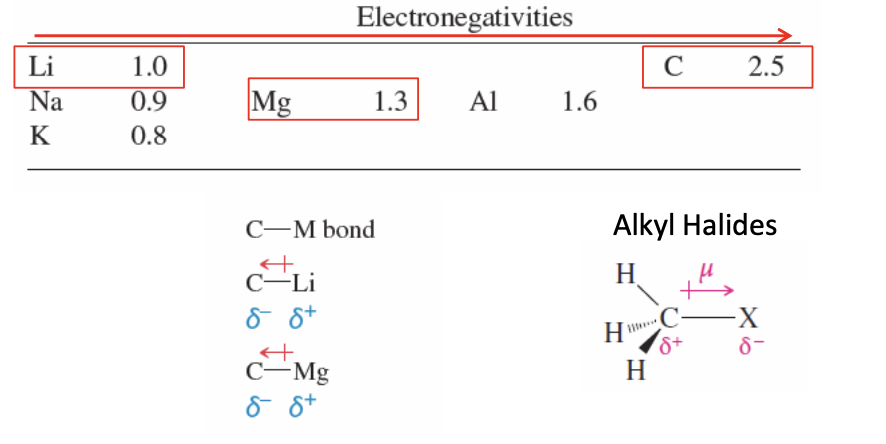
Organometallic Reagents
The bond between a carbon and a metal (ex: C-LI)
carbon is more e- neg than most metals
when the bond is formed, e- density moves towards the cabron
carbon becomes full with e- (becomes partial neg) and the metal lacks e- (becomes partial pos)
carbon w/ partially neg charge allows it to act nucleophilic
carbon ready to attack poor electron atoms (give its e- density away
CARBON IN ORGANOMETALLIC REAGENTS IS NUCLEOPHILIC
Carbon is partial neg, wants to donate e- density
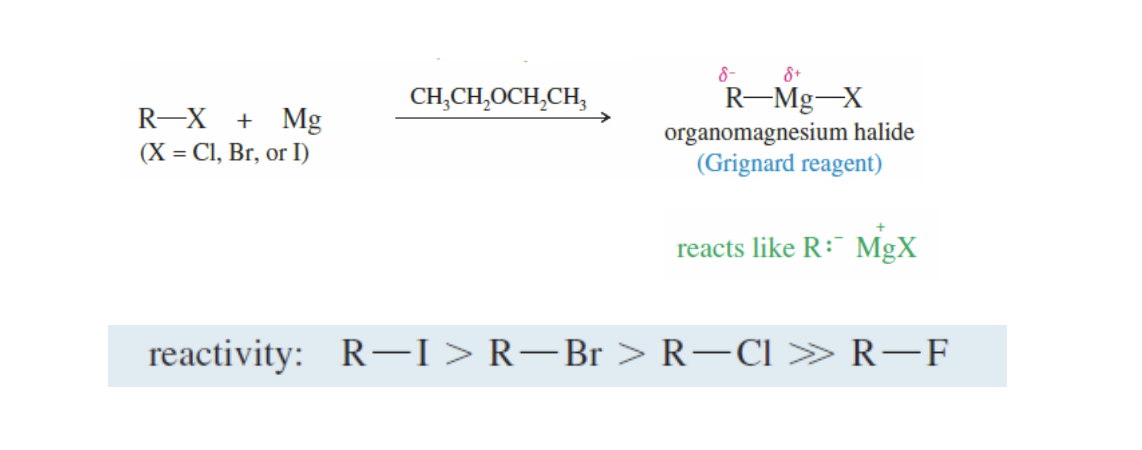
Grignard Reagents
Alkyl Halide (R) - X + Magnesium Metal (Mg) —> ether —> R-MgX
it is stabalized by an ether solvent
The heaver the halide attaches is = the more reactive
heavier halide attached = increased reactivity
Grignard Regeant: R-Mg-X
heaver X = more reactive
The R group can be ANYHALIDE (PRIM, SEC, TERT, VINYL, ARYL
Organolithium Regeanets
Alkyl Halide (R-X) + Lithium (2 Li ) —→ Aprotic solvent —> R-LI + LiX
Regeant: R-Li
can be formed using any type of alkyl halide (R)
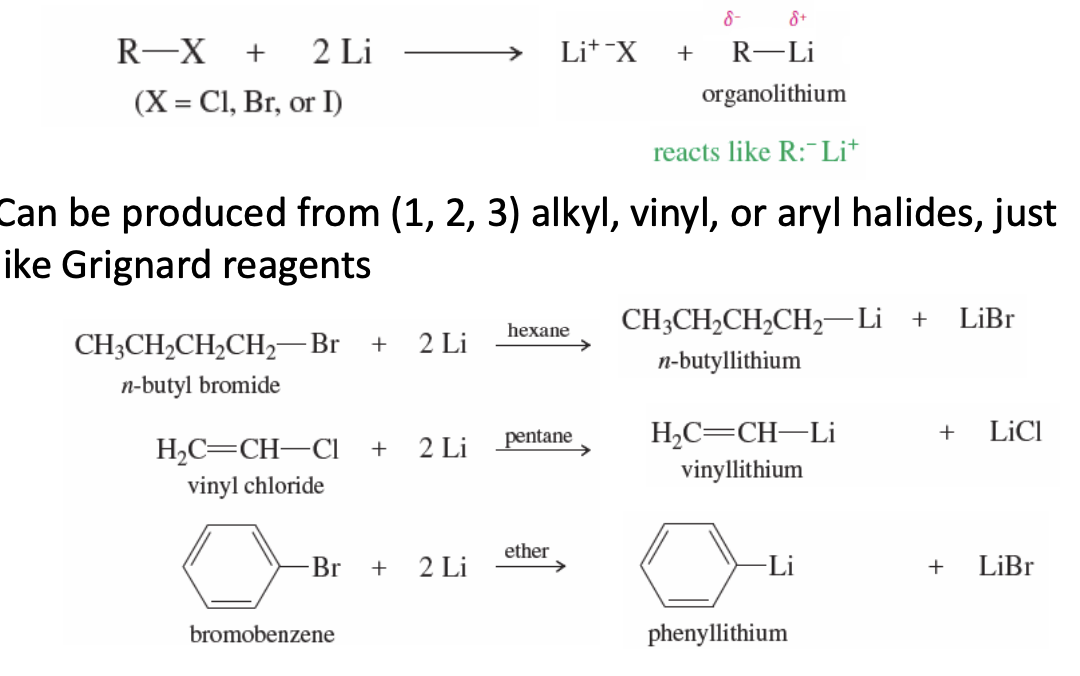
Grignard Reagent Reaction with Carbonyl
Grignard Regenat (R-MgX) + Carbonyl (Ex: Ketone or Aldehyde)
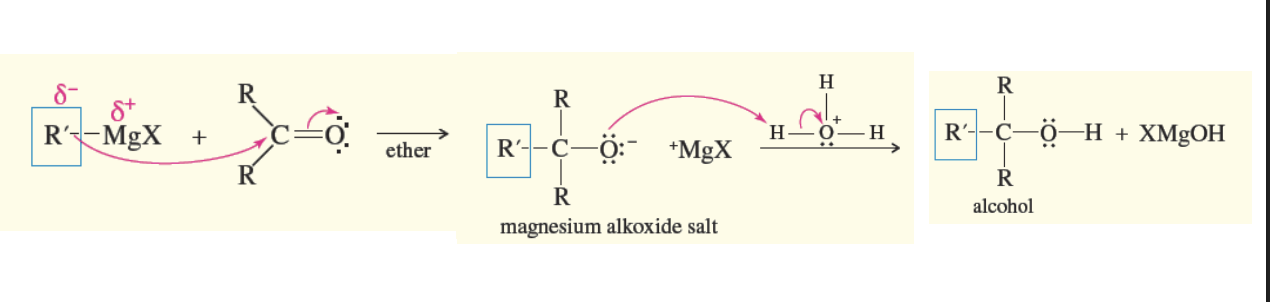
Phenylmagnesium Bromide reacting with carbonyl compound
It is the same mechanism as the grignard regeant reacting with a carbonyl compound
Only now one of the r groups attached is a phenol
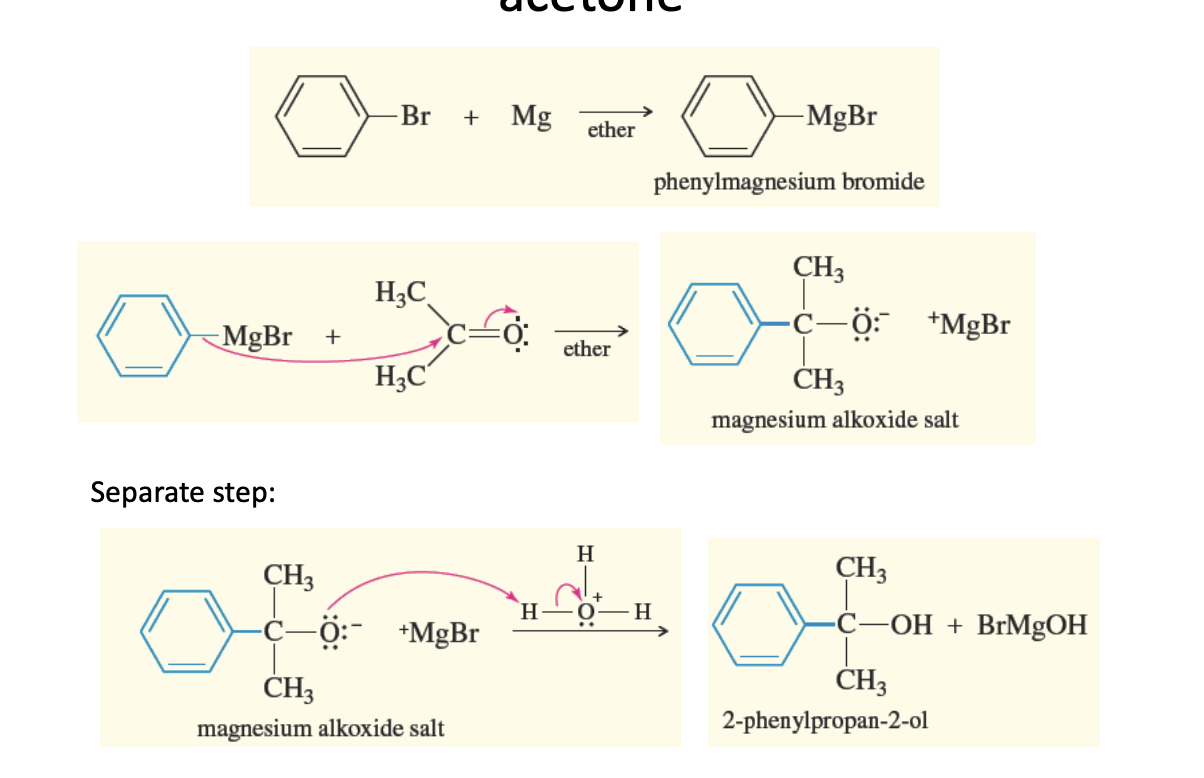
Synthesis of Primary (1° Alcohols)
Overview: Grignard + Carbonyl —> Alcohol
Griganrd + FORMALDEHYDE —> 1° ALCOHOL
1° alcohol = Only one R group (the OH in this synthesis)
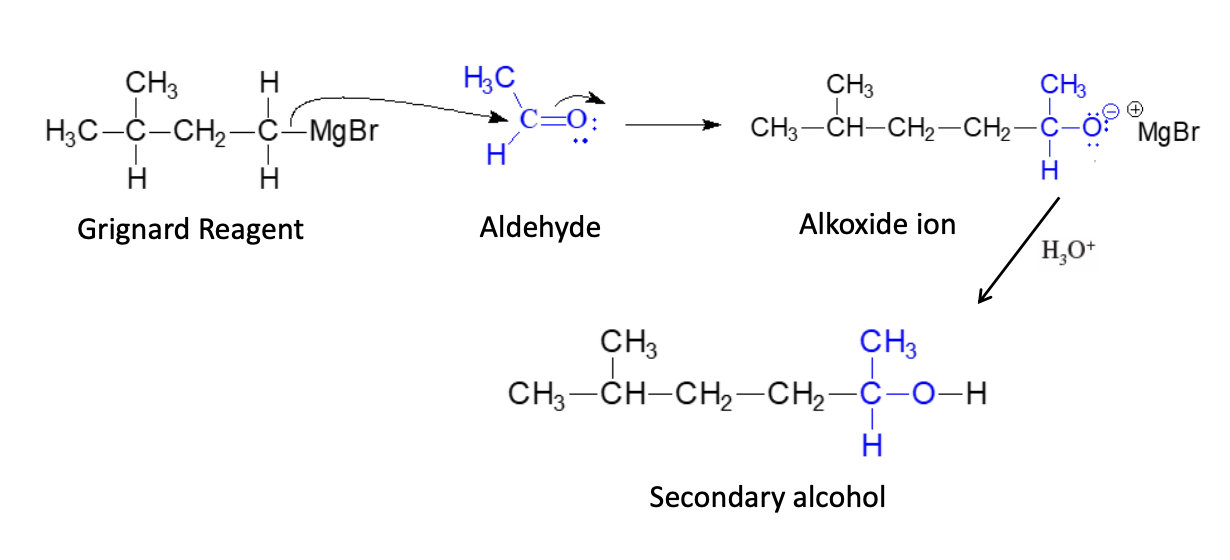
Synthesis of Secondary (2° Alcohols)
Overview: Grignard + Carbonyl —> Alcohol
Grignard + ALDEHYDE —> 2° alcohol
2° alcohol = 2 R groups (CH3 and OH)
ch3 can be any type of R not only ch3
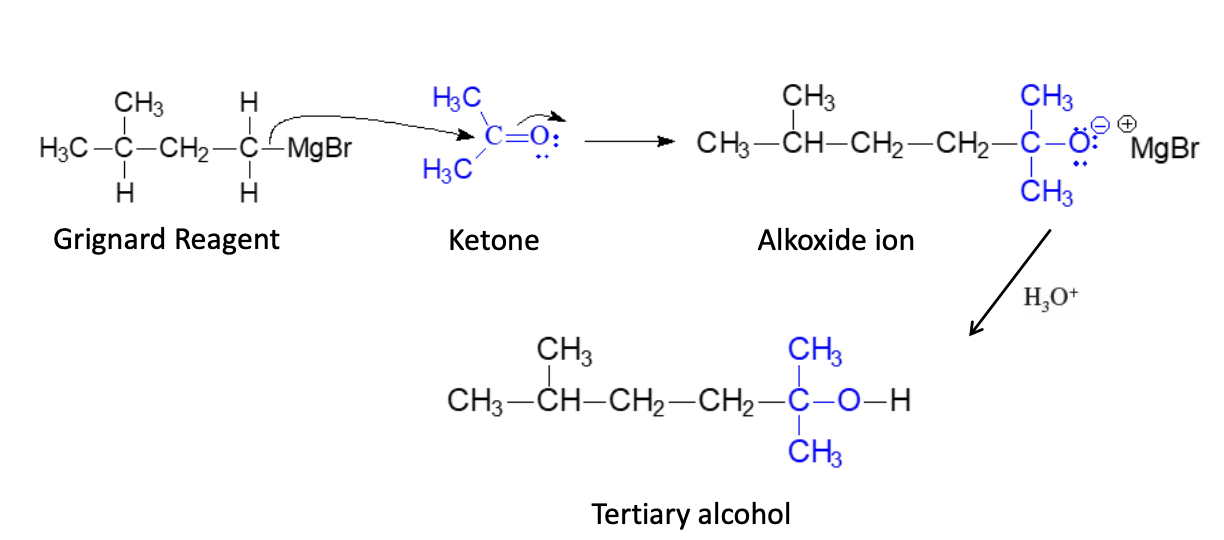
Synthesis of Tert (3° Alcohol)
Overview: Grignard + Carbonyl —> Alcohol
Grignard + Ketone —> Tert Alcohol
3 R groups (Ketone’s Rs and Grignard’s OH)
Grignard Regeant + Acid Chloride
2 Moles of Grignard Regeants are used in this reaction
1 Grig used to start 2nd grig used to react with ketone intermediate
Grid + Acid Chloride —> ether —> Ketone Intermediate
grig +Ketone Intermediate —> H3O+ —> Tert alcohol
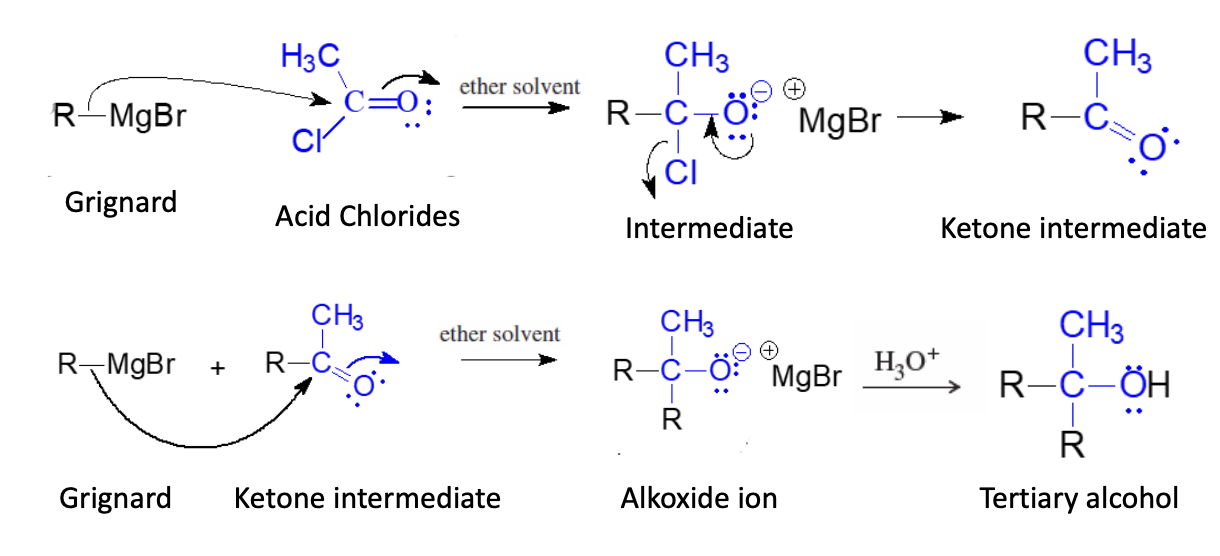
ch3 of acid chloride can be any alkyl halide (r group)
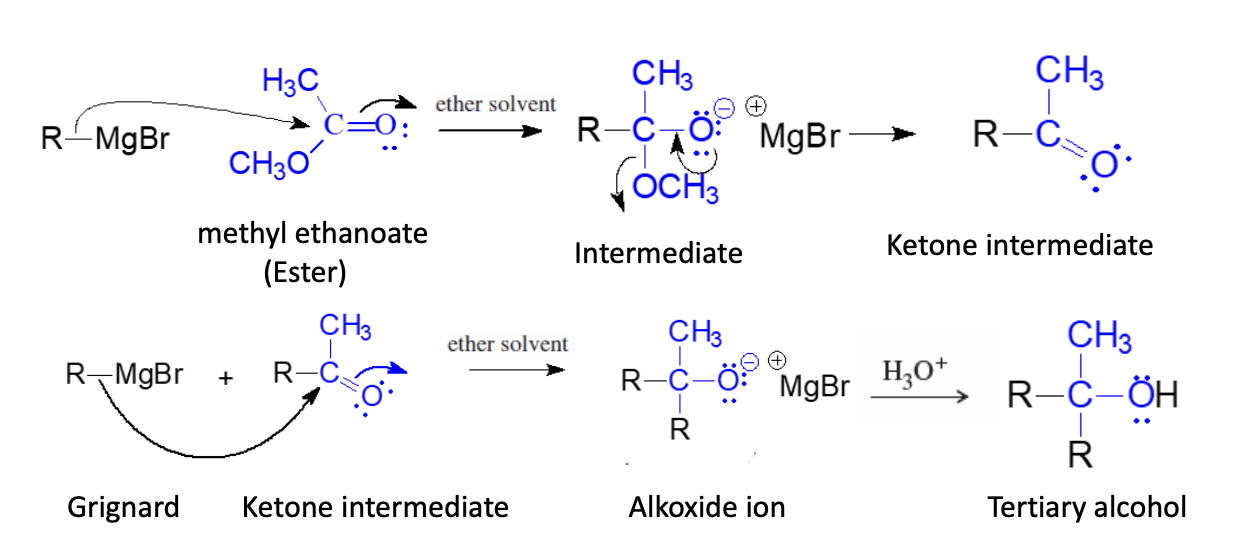
Grignard Regeant + Ester
2 Moles of Grignard Reagent Used
One to Start attack on Ester the 2nd to attack the ketone intermediate
Forms tertiary alcohol
Ehtylene Oxide Ring Opening
Grignard + Ethylene Oxide —> EthyleneoxideOH (Primary alcohol)
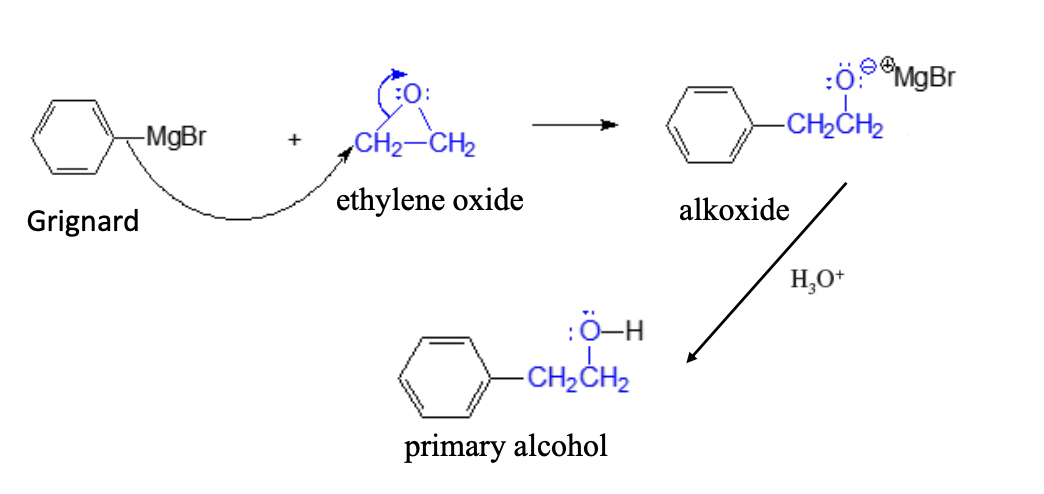
Ring + 2 addition cabrons (OH) product
primary alcohol
Organometallic Reagents Limitations
C-Metal —> The C in the regeant acts as a nucleophile becasue it is more e- neg than most metals
the C wants to donate e- to e-poor species
C-Metal Cant react with acidic protons (H+ donators) and Electorphilic multiple bonds (pos)
With acidic protons —> the C will instantly react to obtain an H+ —> C-Metal becomes a usless alkane
With Mutliple bond speicies—> the speicies are slightly pos which the C wants to react with and destroy the grignard
Acidic Protons (O-H, N-H, S-H),
Electrophilic
Multiple Bonds (C=N, S=O, N=O)
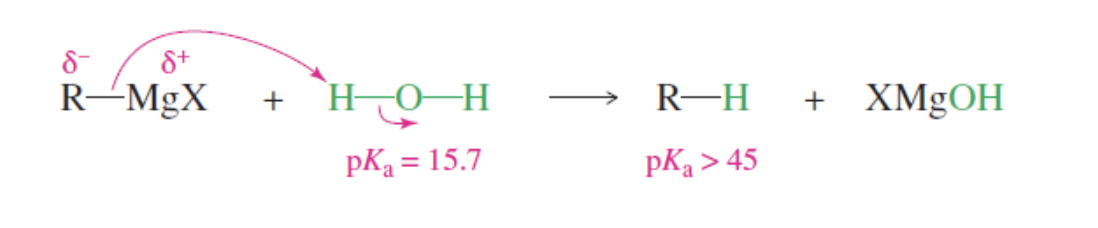
What Hydride Reageants Reduce Carbonyl Groups to 1° and 2 alcohols
lithium alumnum hydride and sodium borohydride
both react in the same manner of Grig reagant + carbonal (attack the c and attaches)
If carbonyl is aldehyde —> 1° alcohol formed
If carbonyl is ketone —> 2° alcohol formed
Both Lithium alumnimum hydride and sodium borohydride attack and attach as an H group
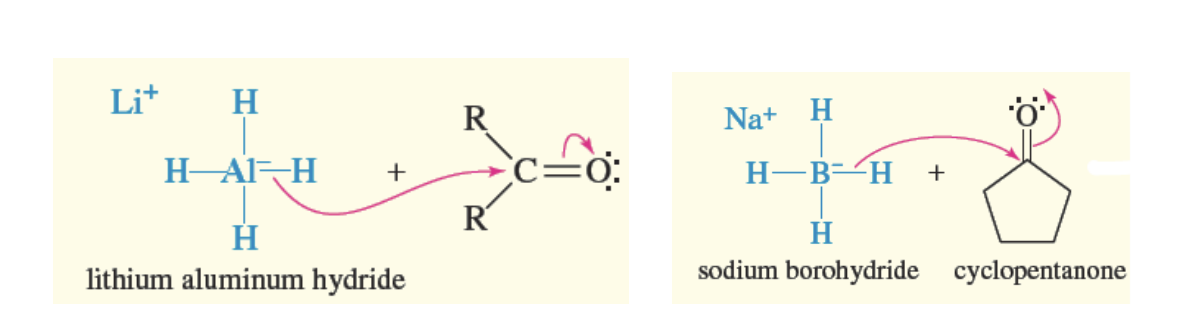
Sodium Borohydride vs Litium Alumnium Hydride as a reducing agent
Sodium Borohydride
only redues ketones and aldehydes carboxyls
weaker reducing agent
Lithium Alumnium Hydride
reduces everything (ketones, aldehydes, esters, and carboxylic acids)
is a stronger reducing agent
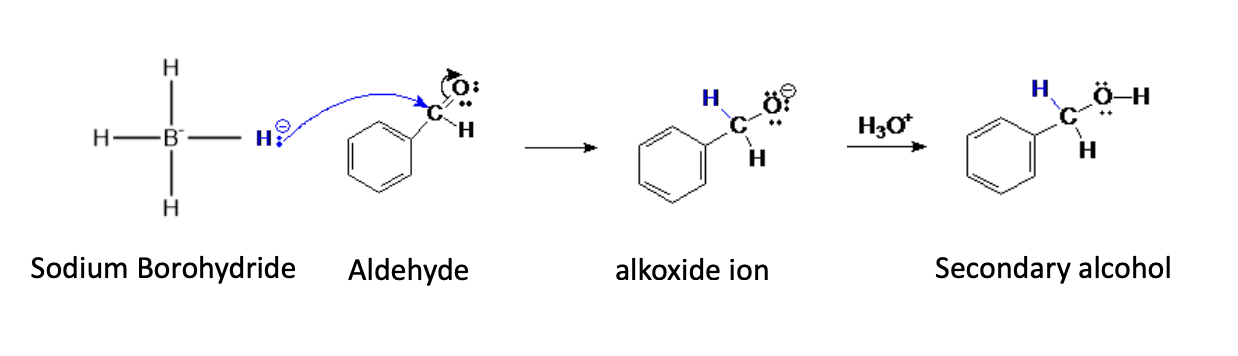
Sodium Borohydride
Weaker Ruducing agent (only reacts with ketones and aldehydes)
When reducing, does the same process as grig +carbonyl but instead of attaching an r grouop it attaches an H+
it also doesn’t use 2 moles
with aldehydes —> 1° alcohol
with aldehydes —> 1° alcohol
with ketones —> 2° alcohol
Lithium Aluminum Hydride
Stronger reducing agent (reduces everything: ket, alde, esters, & carboxylic acids)
alde —> 1° alcohol
ket —> 2° alcohol
esters & carboxylic acids —> 1° alcohol
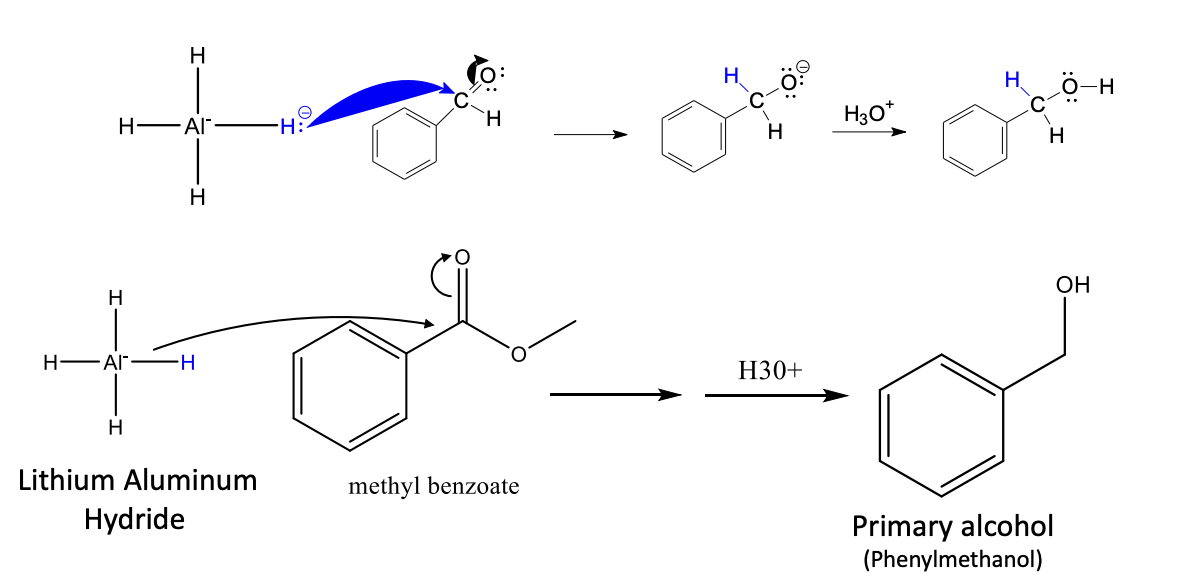
Same process as grig + carbonyl (but instead of attaching R it attaches an H+)
it also doens’t use 2 moles
Catalytic Hydrogenation
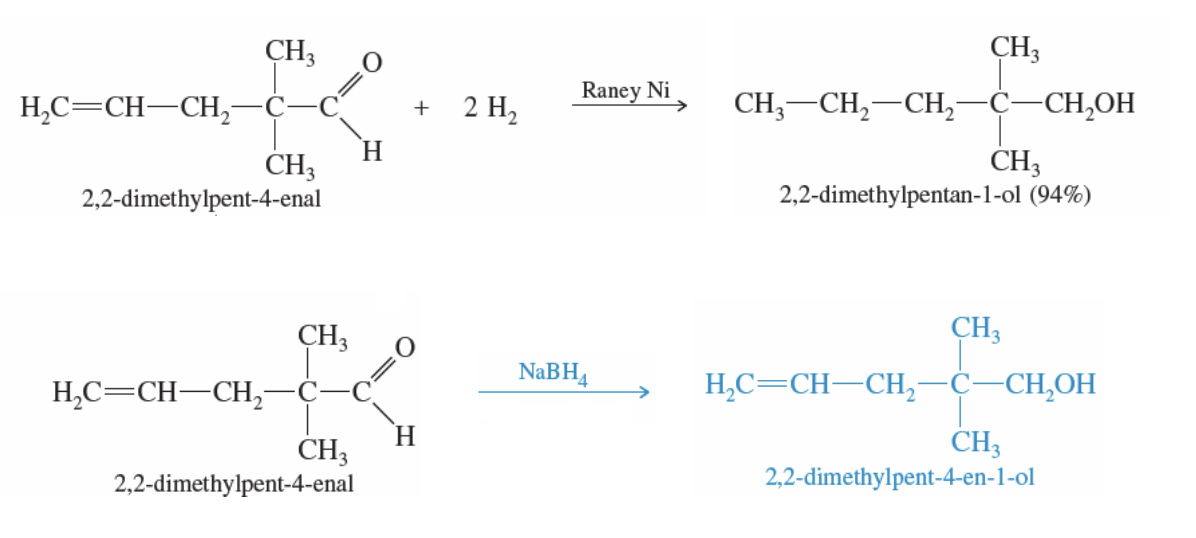
Ket or alde + 2H2 + Acid Catalyst —> reduces to a 2° or 1° alcohol
reduces C=O bond
and C=C bond
Ket or alde + NABH4 —> reduces to a 2° or 1° alcohol
reduces C=O bond
Thiols
Configuration where SH is written instead of OH
sulgar analogues (S—> O)
They are more acidic than alcohols (lower pka than alcohols)
Phenol > Thiols > Alcohols in terms of acidity
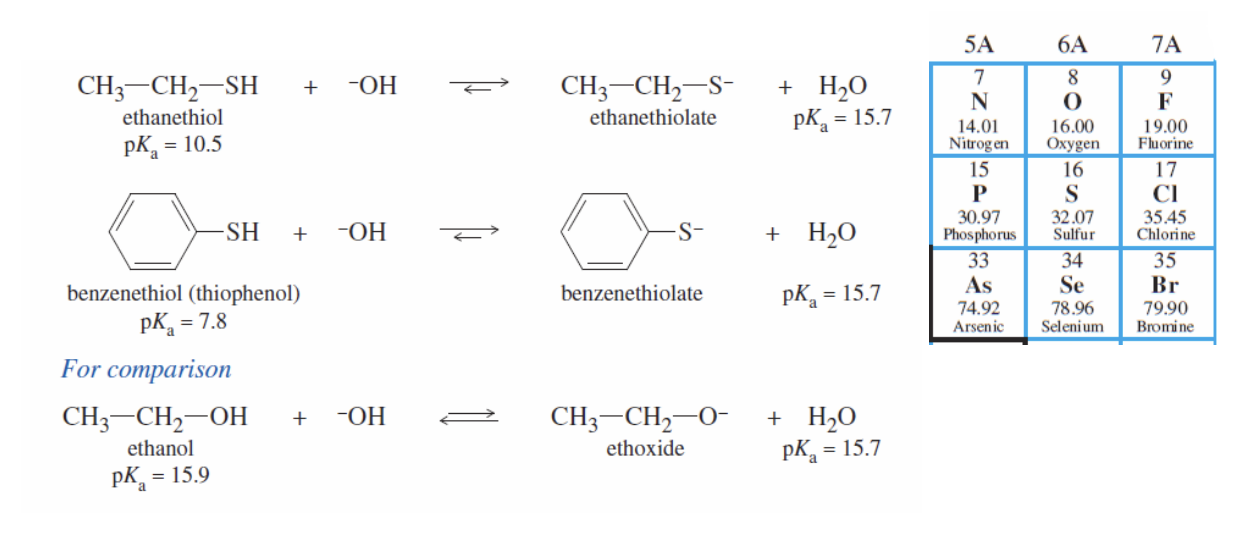
IUPAC: written on the end of an alkane (ex: ethanethiol)
Thiol Synthesis
NaSH + RX —> R-SH + NaX
Na+ SH reacts with a primary alkyl halide (unhindered, RX)
Produces the thiol: R-SH)
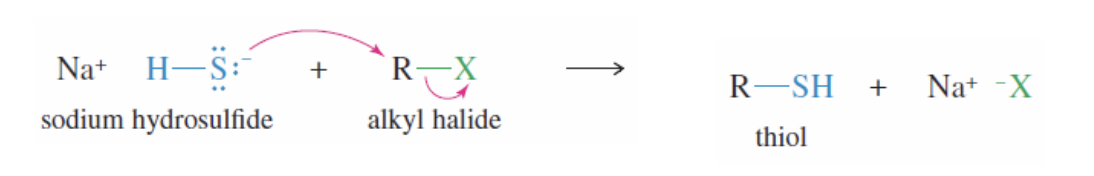
Thiol Oxidation
Thiol: R-SH
It is easily oxidized into disulfides (R-S-S-R)
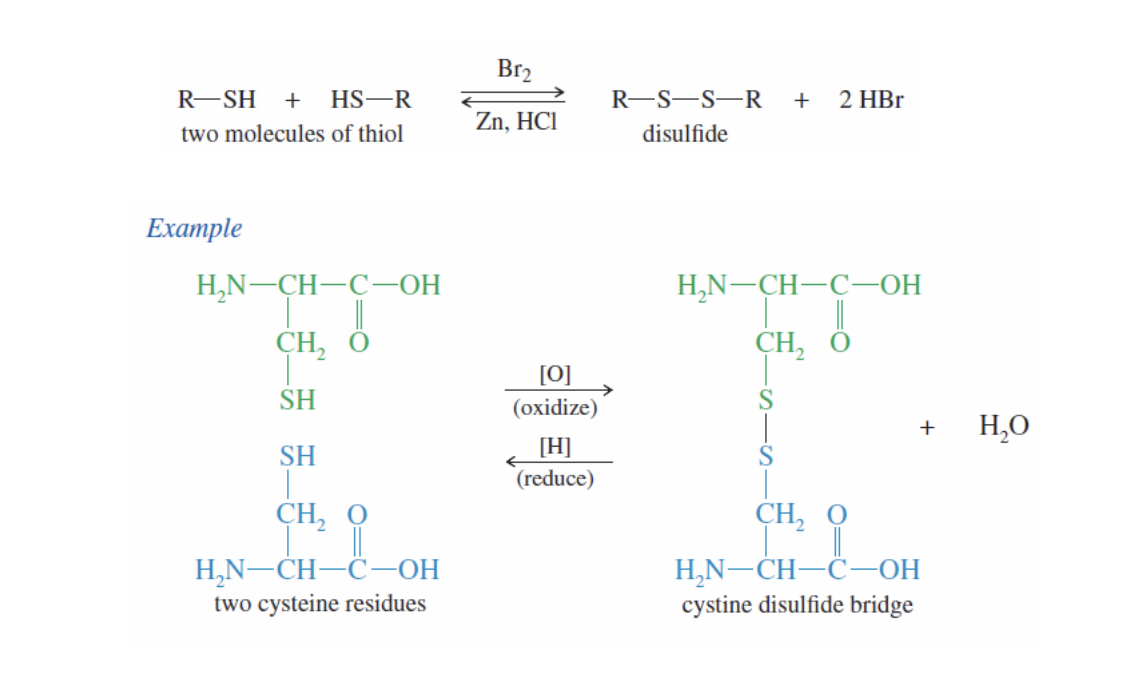
2 Molecules of Thiol + Oxidizing agent —> R-S-S-R
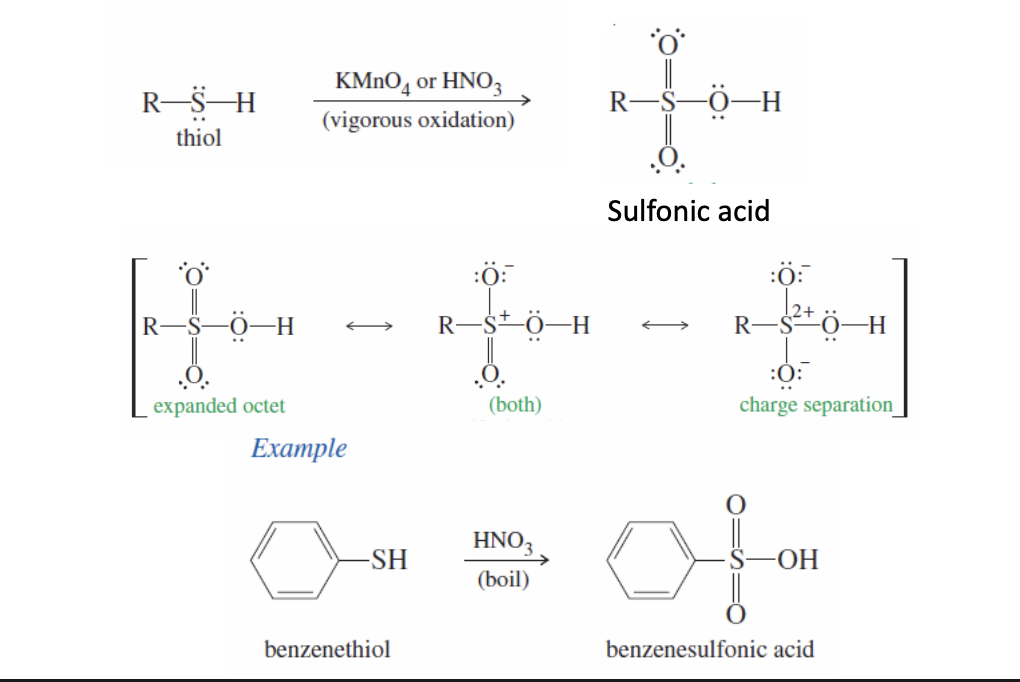
Thiol Oxidation to Sulfonic Acid
Thiol (R-SH) can be oxidized via KMnO4 or HNO3 into a sulfonic acid
results in an expanded octet (the sulfonic acid) —> that can then show charge separation (+ and - charges)