Chapter 1: Structure and Bonding - Organic Chemistry
1/69
Earn XP
Description and Tags
Flashcards cover Chapter 1 topics: organic chemistry fundamentals, atomic structure, isotopes, bonding (ionic and covalent), Lewis structures, electronegativity and polarity, hybridization (SP3, SP2, SP), molecular geometry, and bond properties.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
70 Terms
What is Organic Chemistry?
branch of chemistry for carbon-based molecules
Besides carbon, which elements are commonly found in organic compounds according to the notes?
Hydrogen, Oxygen, Nitrogen, Sulfur, Phosphorous, Chlorine, Fluorine, Bromine, Iodine, Lithium, Sodium, Magnesium, Copper, and Zinc
What is a carbon skeleton?
The chain or ring of carbon atoms in an organic molecule
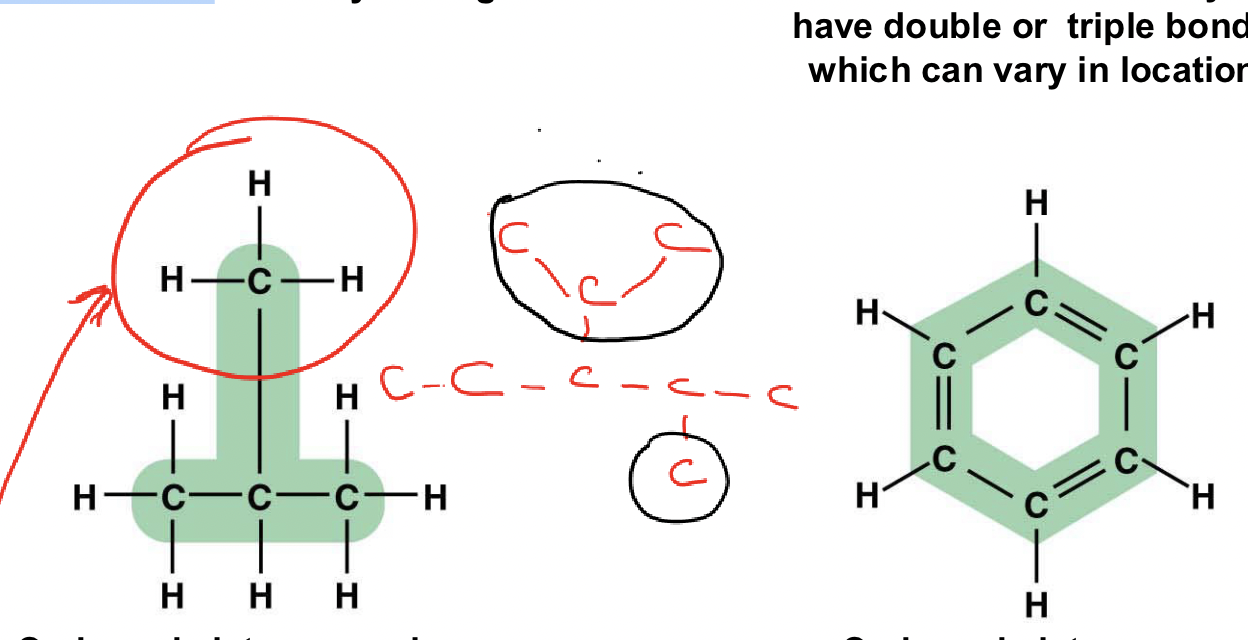
What types of bonds can Carbon form with other atoms?
Single, double, and triple bonds
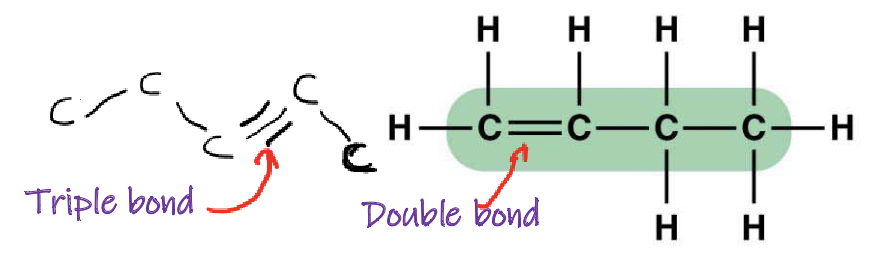
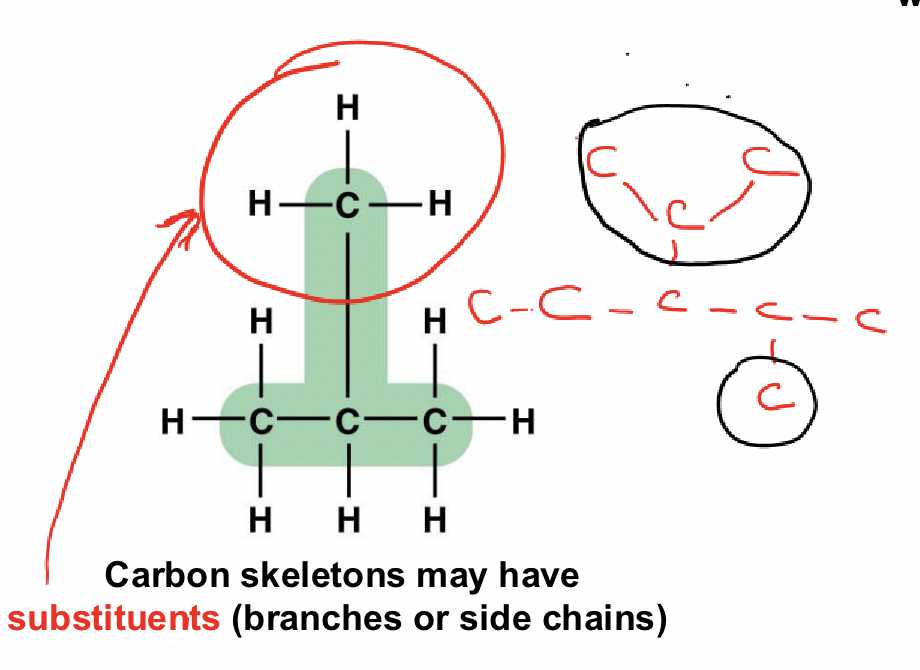
What are substituents?
Side chains attached to the carbon skeleton
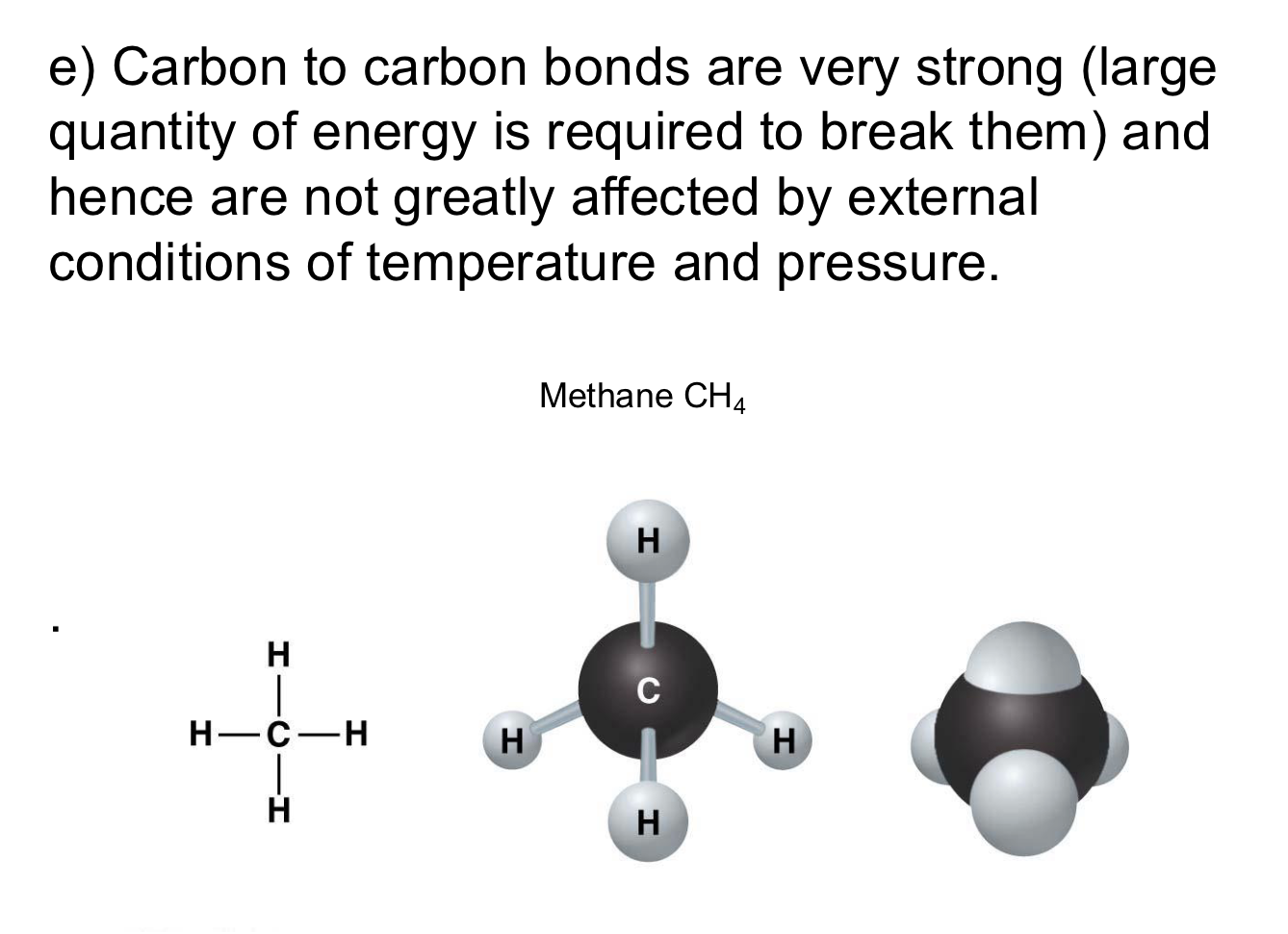
Why are carbon carbon bonds important?
They are very strong and hard to break because they require lots of energy
What is the Bohr model?
A model with electrons in shells around the nucleus
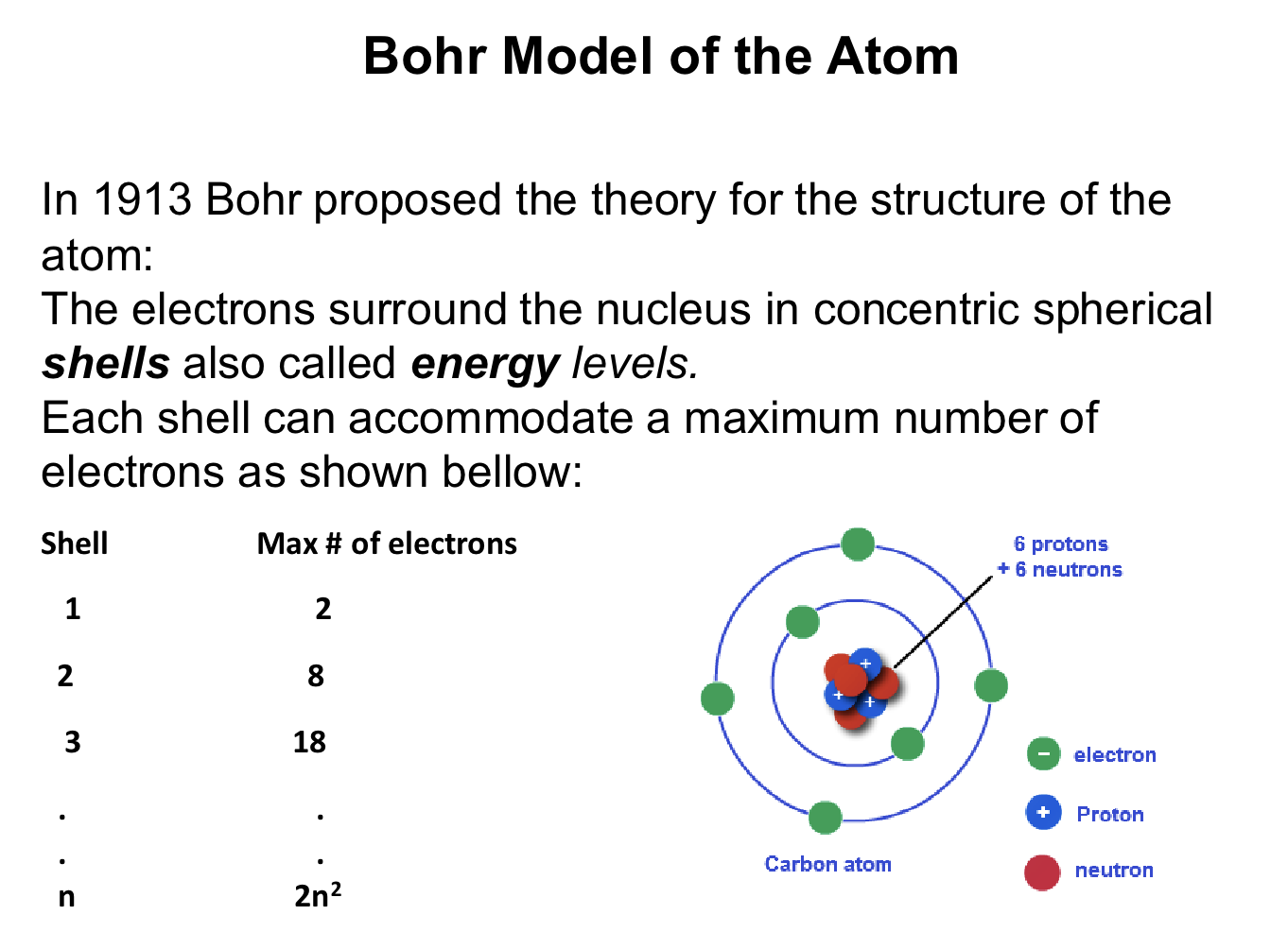
What is the valence shell and why is it important?
The outermost electron shell with valence electrons that participate in bonding and determine reactivity
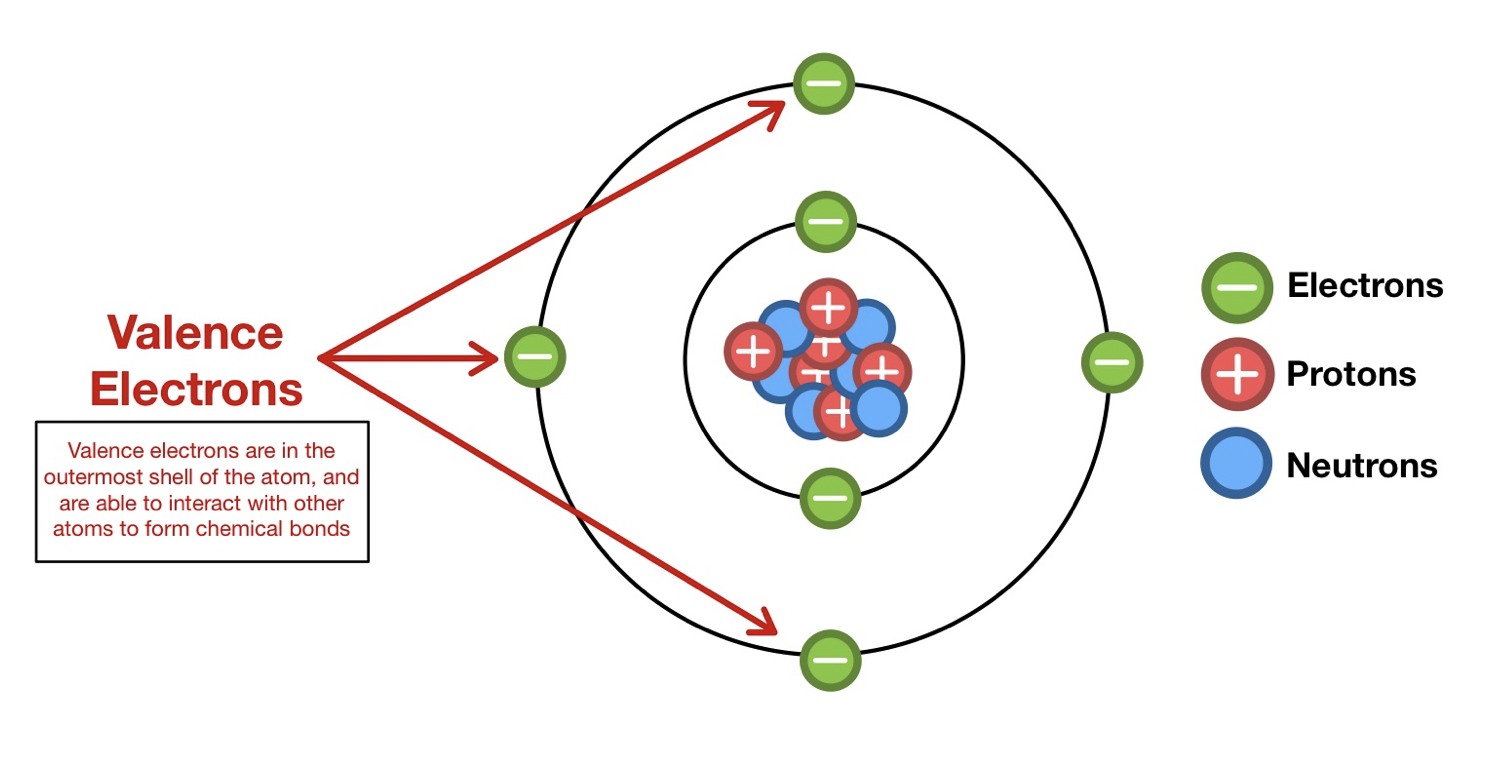
What are isotopes?
Atoms of the same element with the same number of protons but different numbers of neutrons, which gives different mass numbers
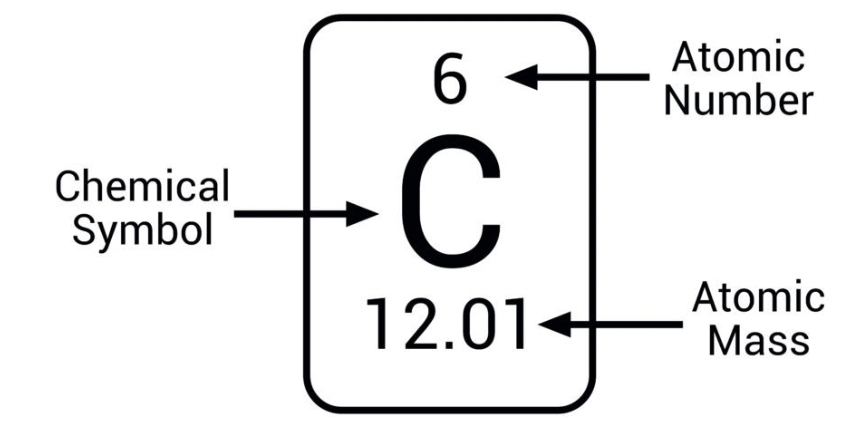
What is the atomic number? What does it refer to?
number of protons in the nucleus in a neutral atom, equals the number of electrons
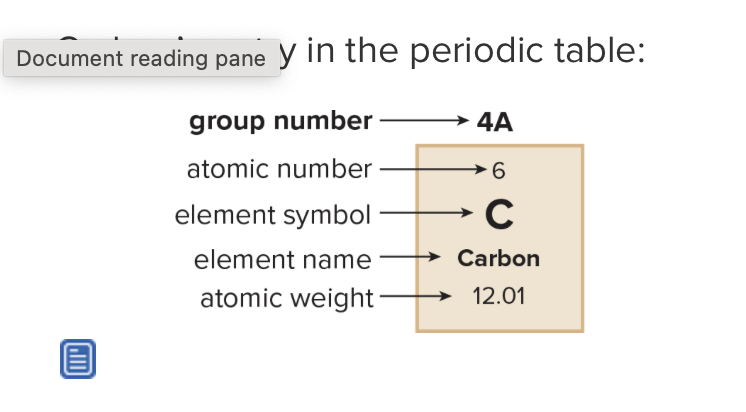
What is the mass number?
Sum of protons and neutrons in nucleus
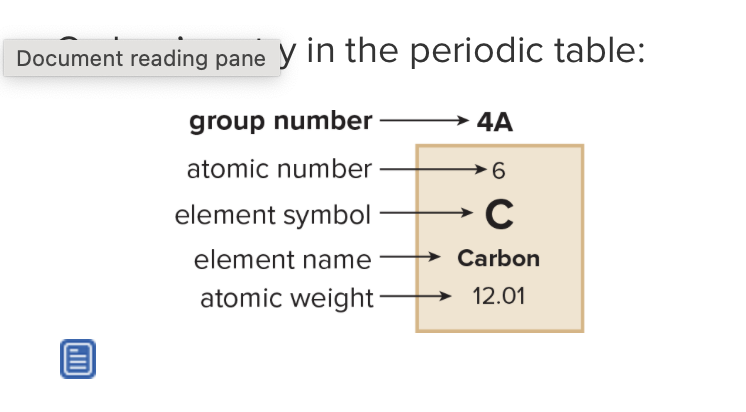
What is the atomic weight?
weighted average of masses of all isotopes of an element
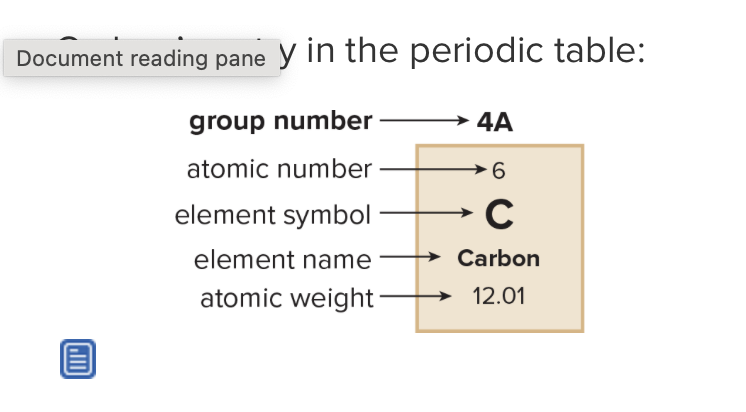
What is an ion? Cation? Anion?
A charged atom
Cation is positively charged with more protons than electrons
Anion is negatively charged with more electrons than protons
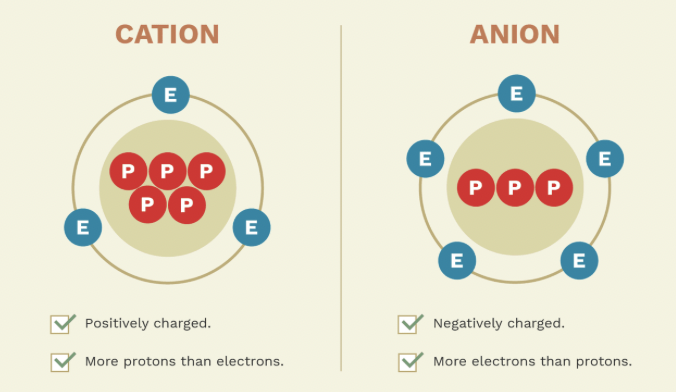
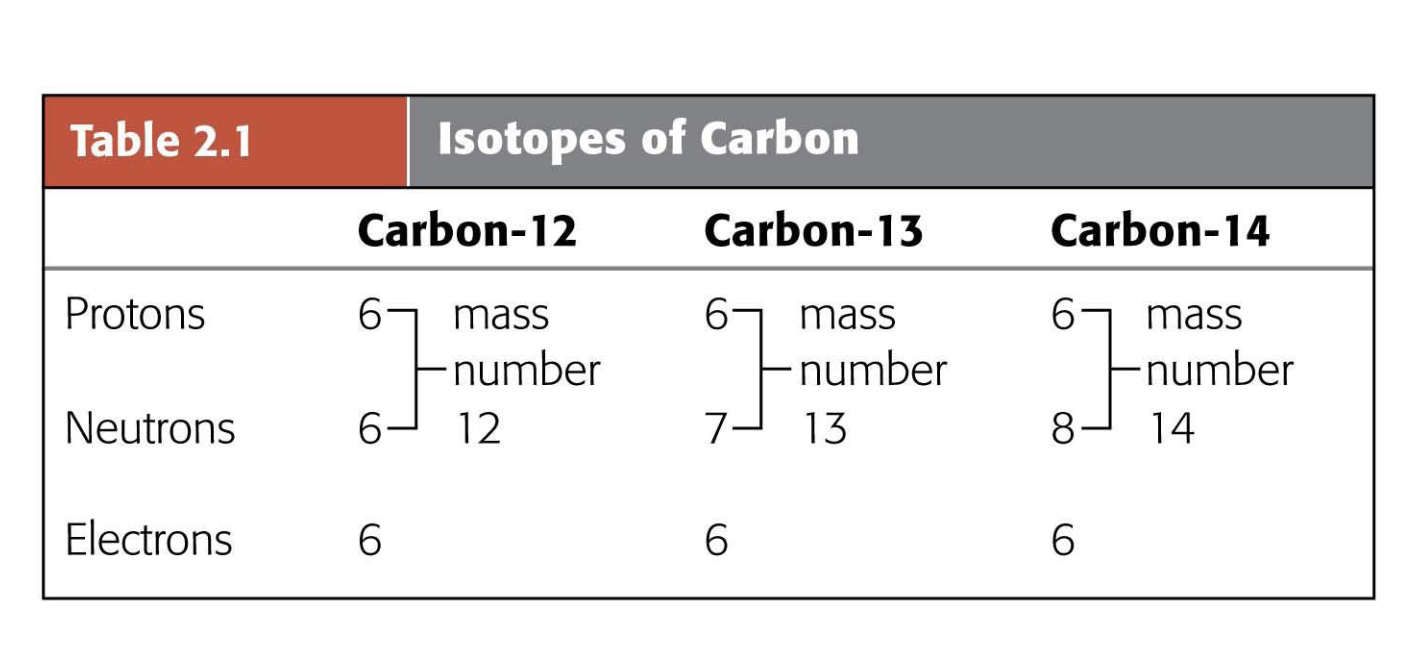
What are isotopes of carbon, and what are their mass numbers and neutron counts?
Carbon-12 (6 protons, 6 neutrons), Carbon-13 (6 protons, 7 neutrons), Carbon-14 (6 protons, 8 neutrons)
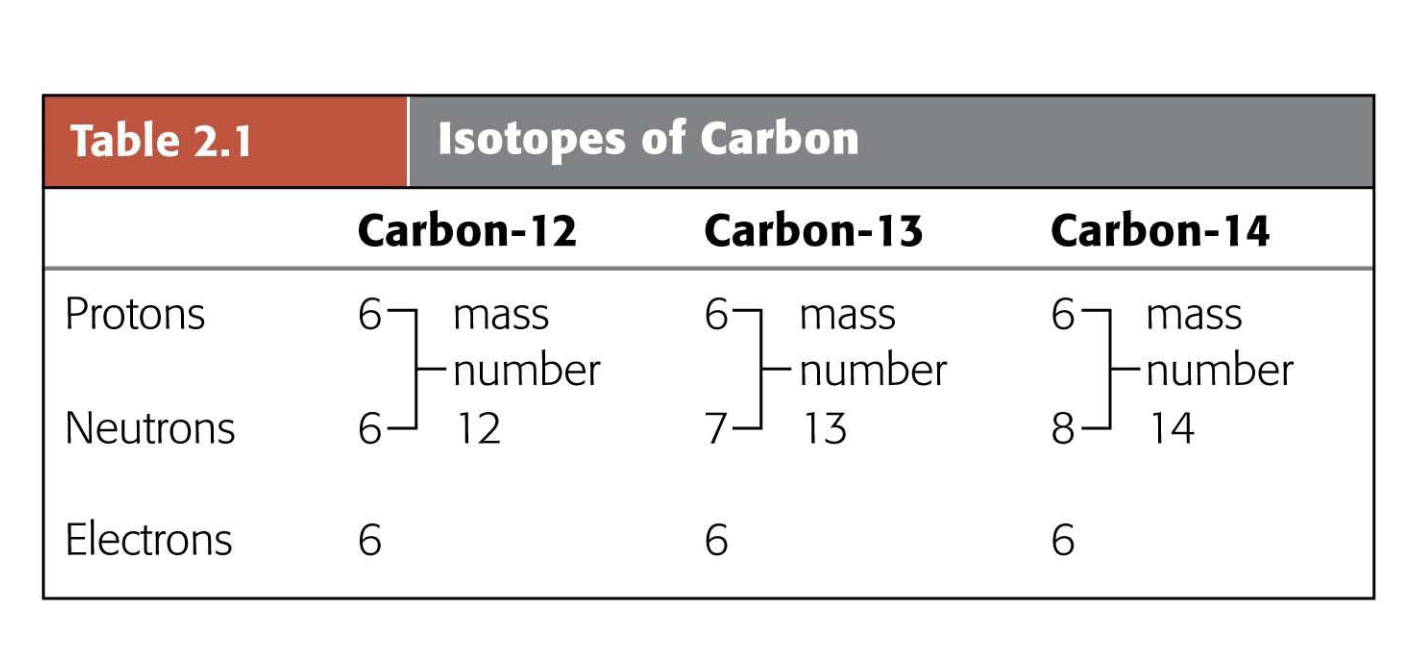
What is the importance of atomic number for Carbon?
Carbon has 6 protons
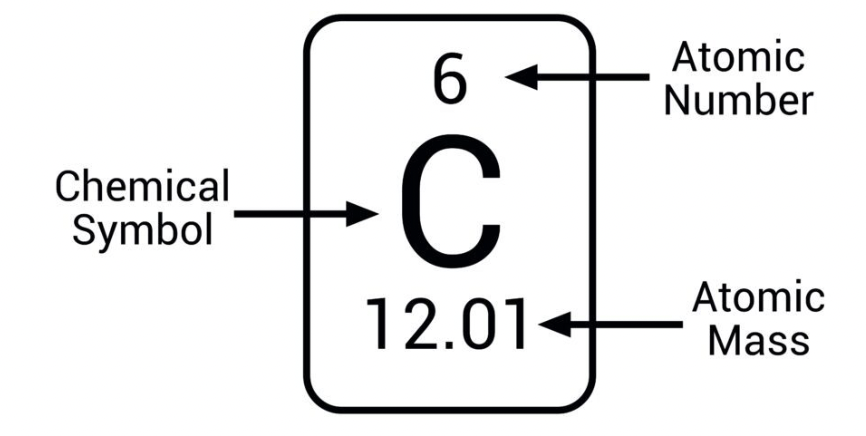
What is electron configuration and which principles govern it?
Arrangement of electrons in orbitals; governed by Aufbau principle, Pauli exclusion principle, and Hund’s rule

What is the Aufbau principle?
Lower-energy orbitals are filled first

What is the Pauli exclusion principle?
No more than two electrons may occupy the same orbital with opposite spins
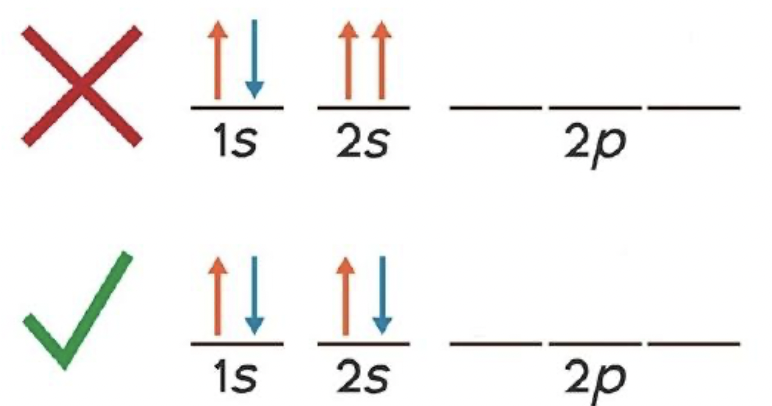
What is Hund’s rule?
When filling orbitals of equal energy, electrons occupy separate orbitals with parallel spins before pairing in the same orbital

What is a Lewis dot symbol?
The symbol of an element with its valence electrons shown as dots around the symbol, with up to four sides filled.

What are noble gases and why are they often described as inert?
Gases in group 8 with stable, fully paired valence electrons; have little tendency to form bonds.
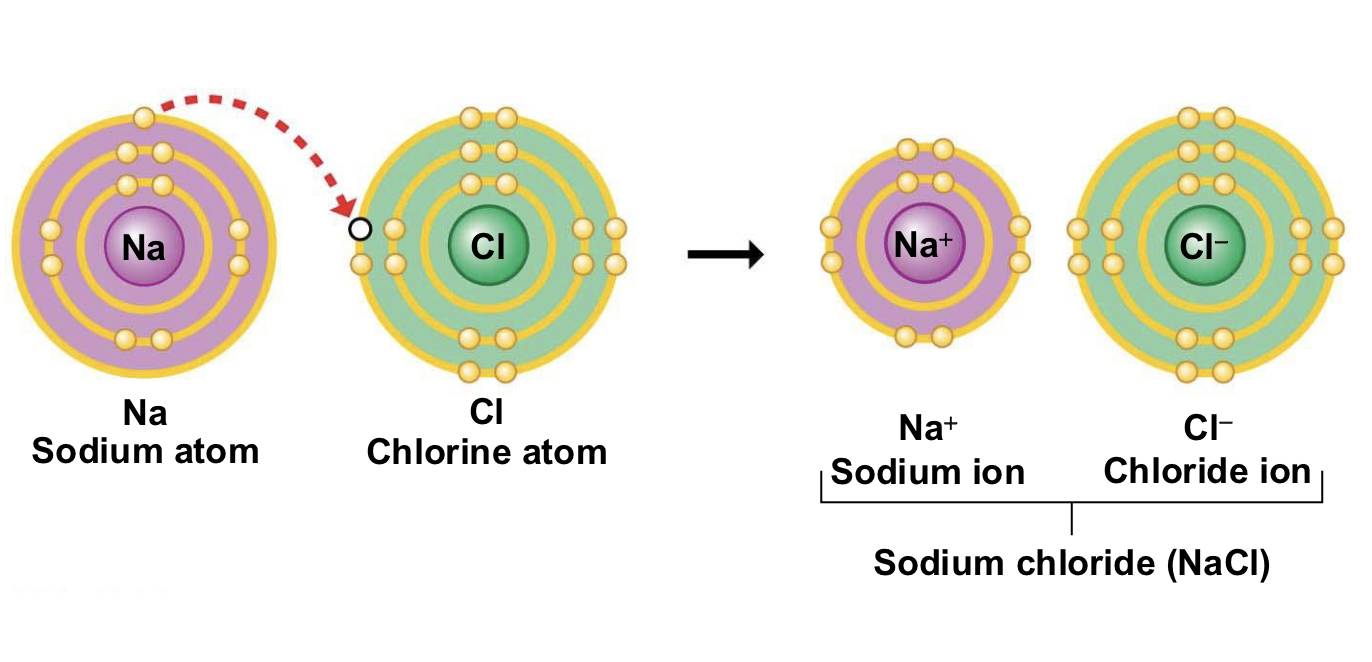
What is an ionic bond? Give an example of a typical ionic bond
A bond formed by the transfer of electrons from one atom to another, creating cations and anions held together by electrostatic attraction.
Typically occurs when element from group 1 (far left) combines with a nonmetal from group 7 (far right), such as sodium chloride (NaCl).
What is a covalent bond? Give an example of a typical covalent bond
A bond formed by sharing electrons between two nuclei.
It typically occurs between nonmetals and allows atoms to achieve filled valence shells.
An example of a typical covalent bond is the bond between two hydrogen atoms in a hydrogen molecule (H₂).

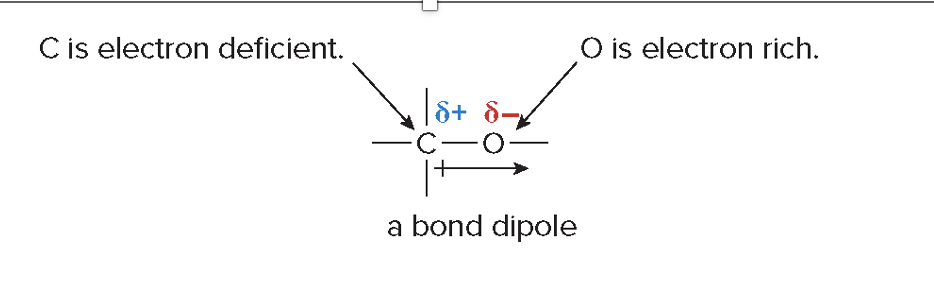
How can you determine bond polarity and dipoles?
By comparing electronegativity values to identify polar bonds and then assessing the geometry to see if bond dipoles reinforce or cancel.
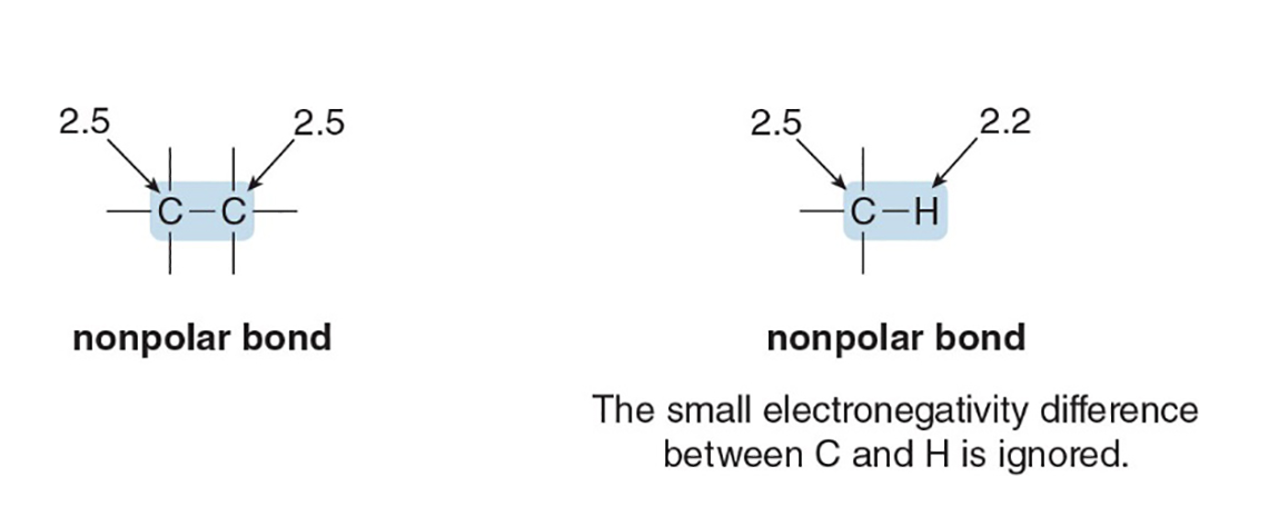
What is electronegativity?
An atom’s attraction for electrons in a bond; influences bond polarity.
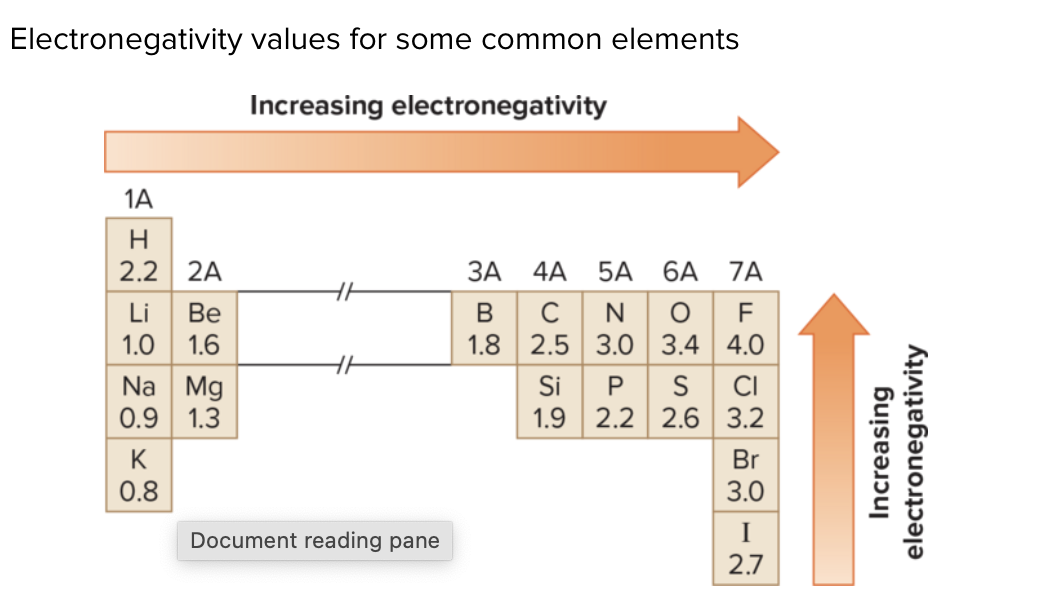
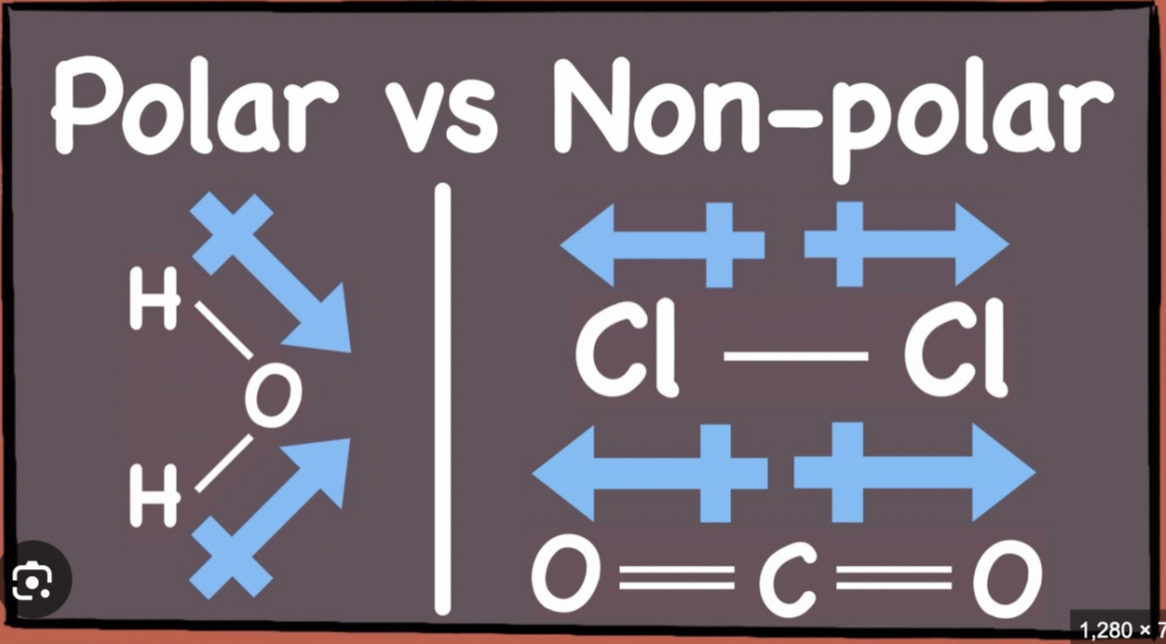
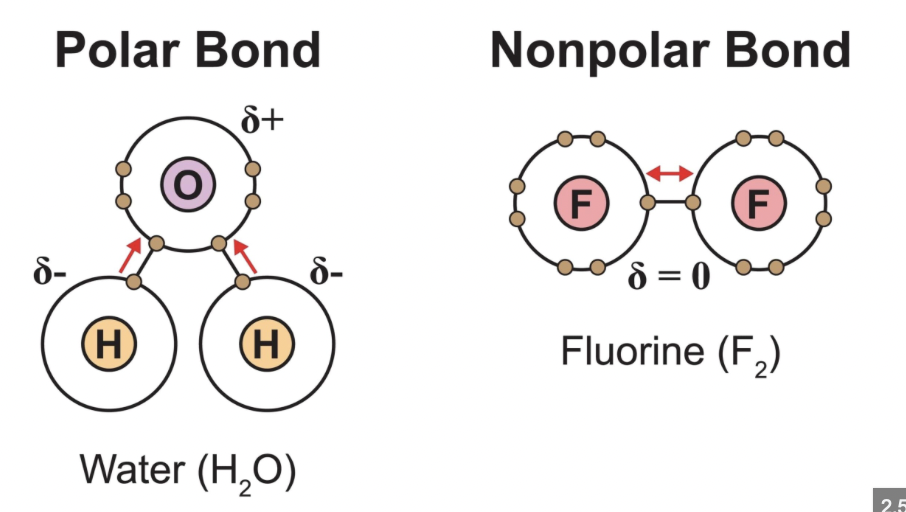
What distinguishes polar bonds from nonpolar bonds?
Polar bonds arise from electronegativity differences, giving dipoles; nonpolar bonds have little or no difference in electronegativity leading to even electron distribution.
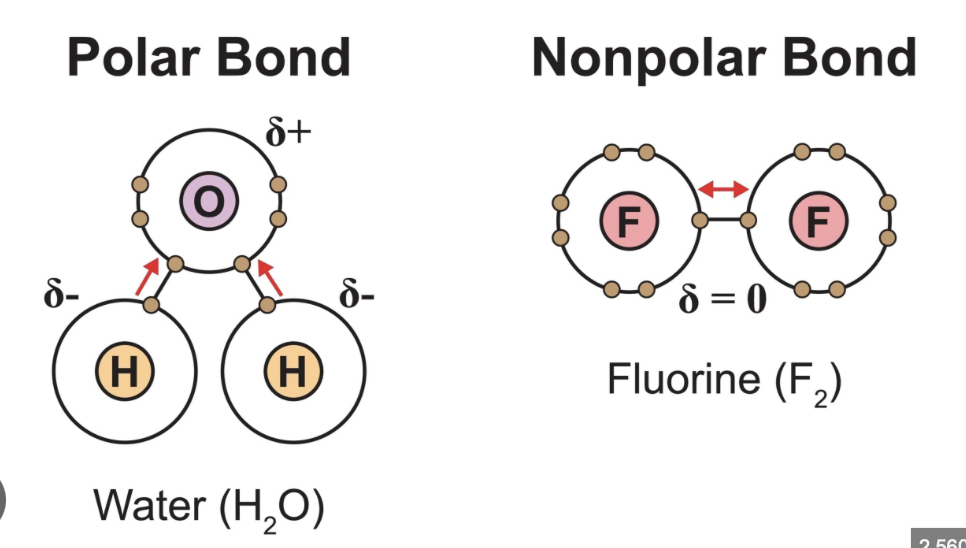
What is a dipole moment?
A measure of the separation of positive and negative charge in a molecule due to bond polarity.
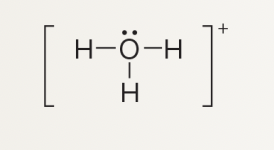
How is formal charge calculated in a Lewis structure?
Assign electrons owned by an atom as its lone pairs plus half of shared electrons; compare to the atom’s valence electrons.
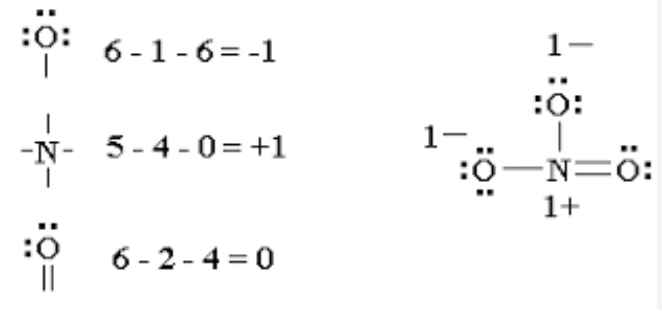
What is homolytic bond cleavage?
Bond cleavage where each fragment takes one electron, producing two neutral atoms or radicals.
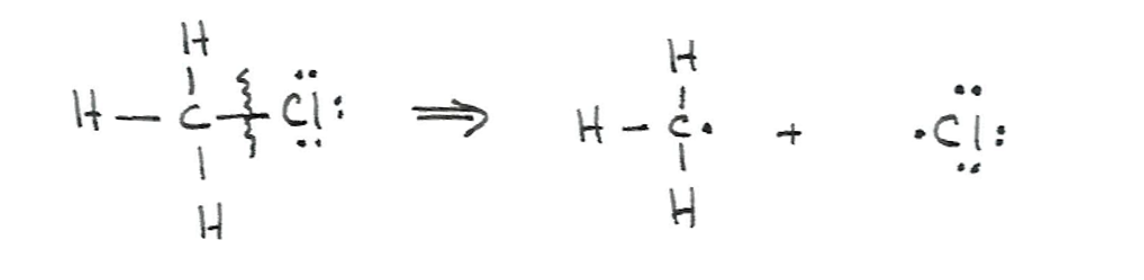
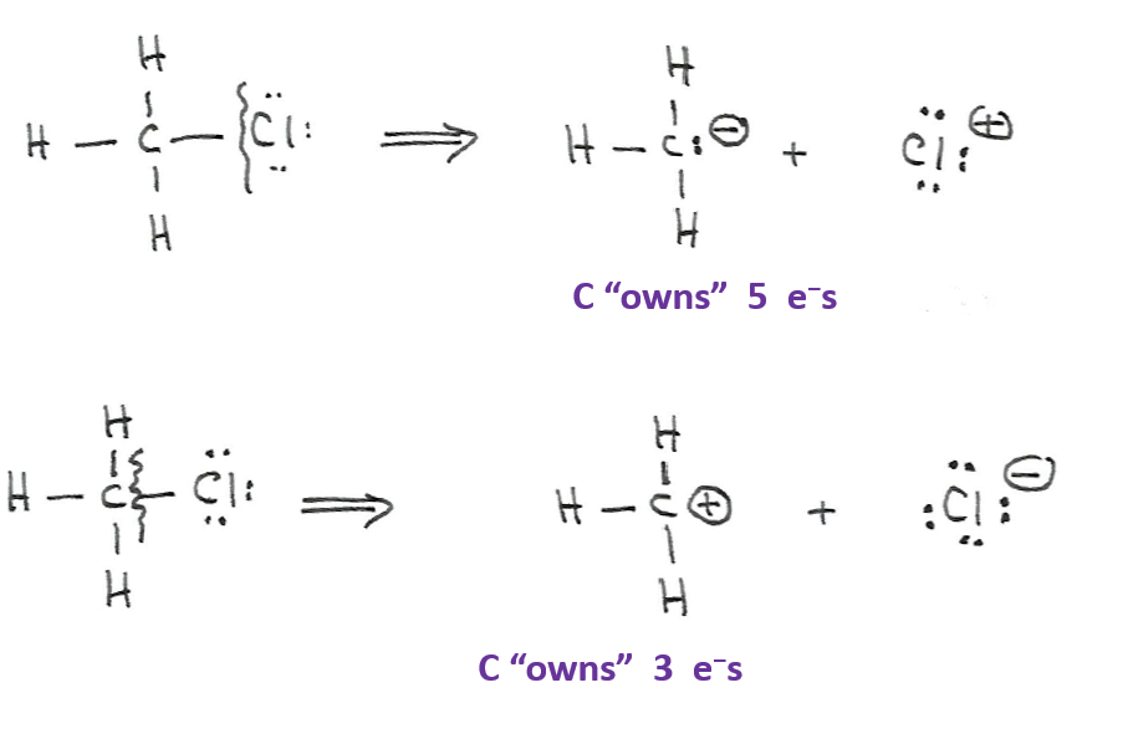
What is heterolytic bond cleavage?
Bond cleavage where one fragment takes both electrons, producing an anion and a cation with formal charges.
What are sigma and pi bonds? Does a double bond have one or both?
Sigma (σ) bonds arise from head-on overlap of orbitals
pi (π) bonds arise from sideways overlap of p orbitals
A double bond consists of one σ and one π bond.
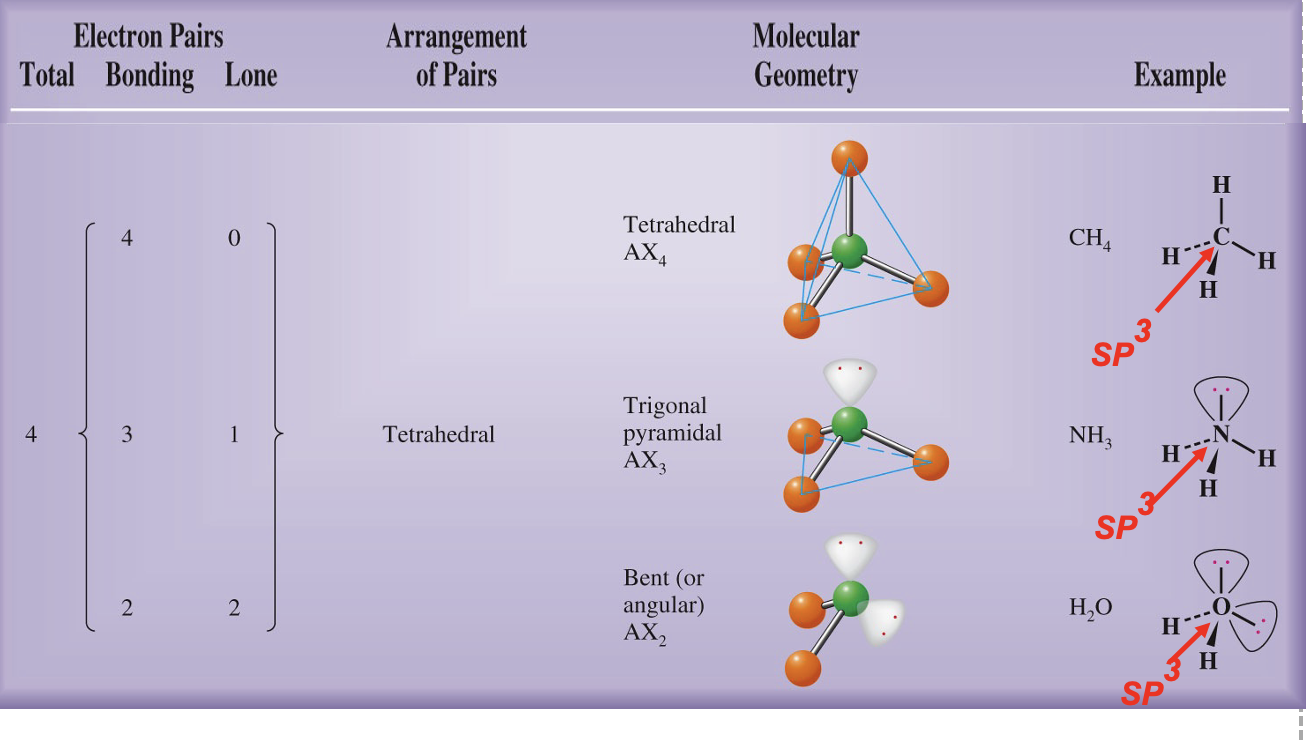
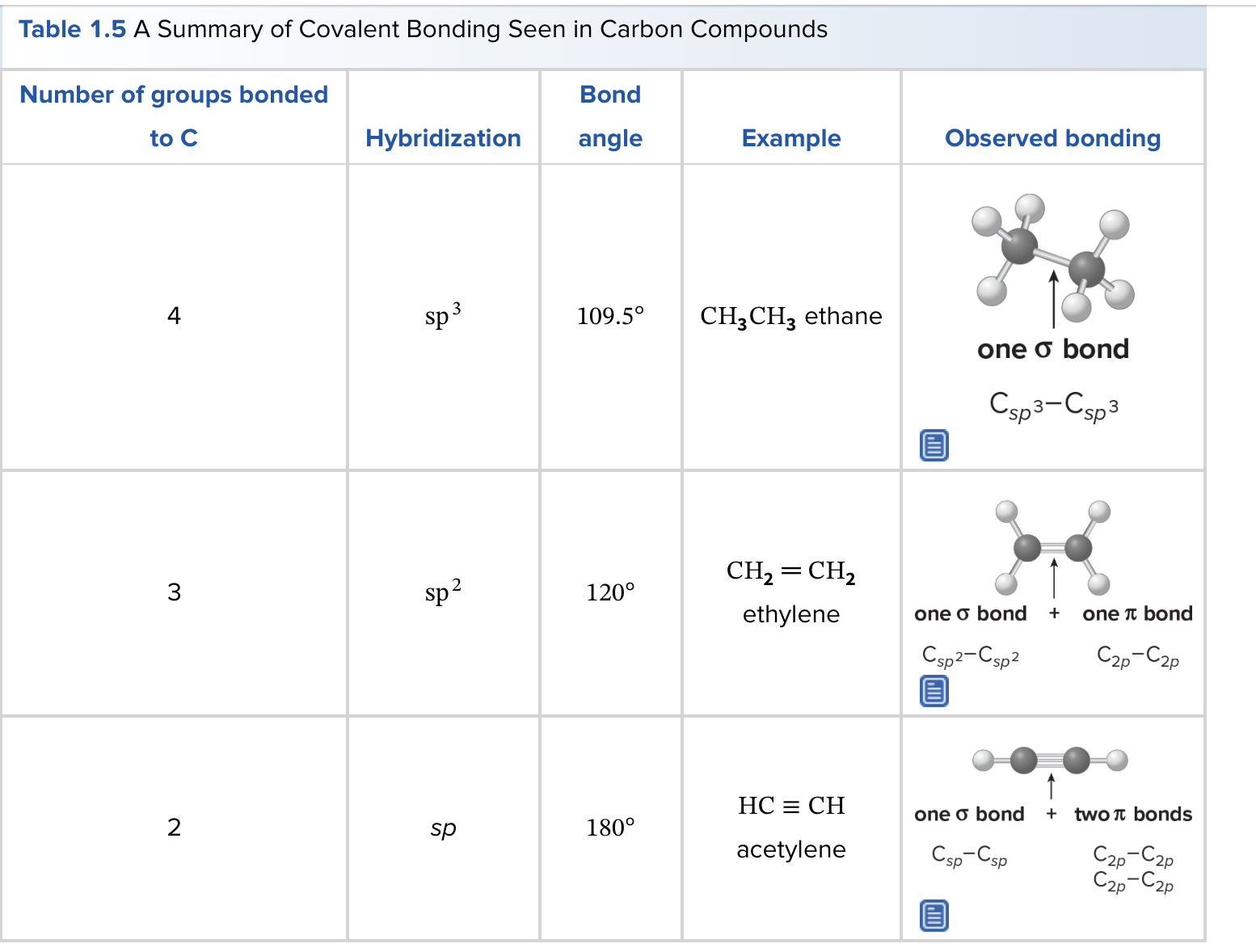
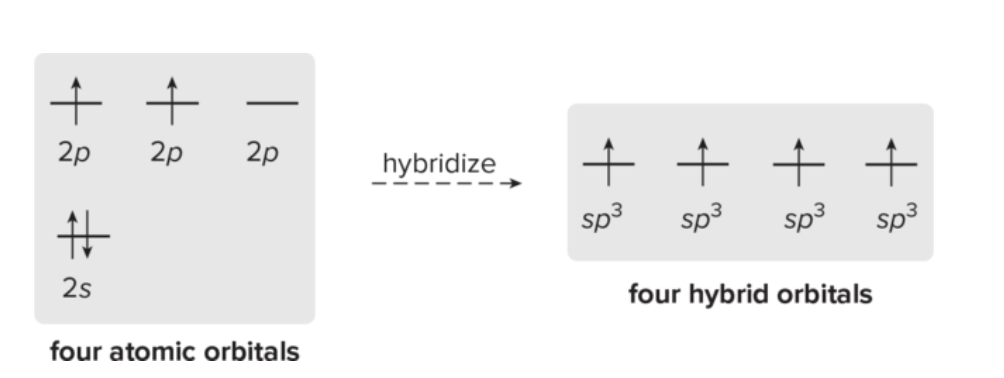
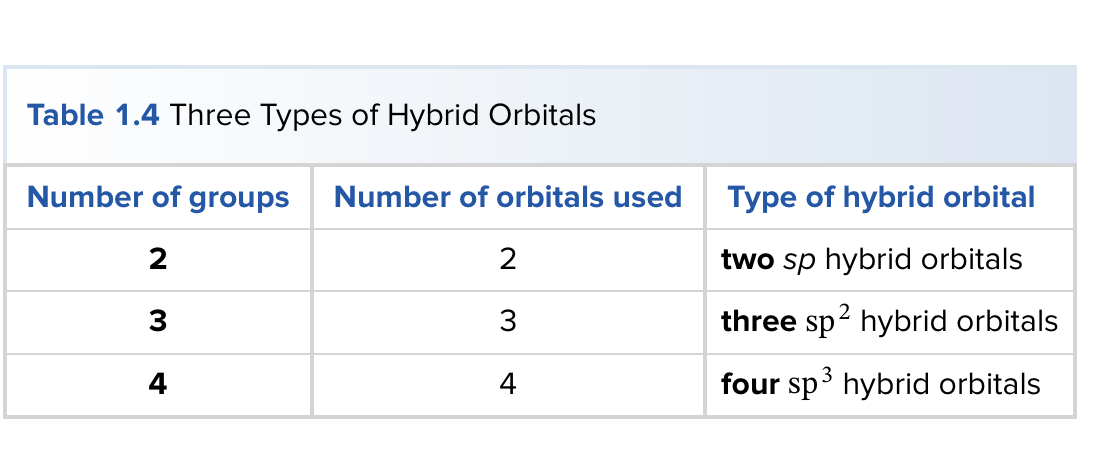
What is carbon hybridization SP3 and its geometry?
SP3 hybridization forms four equivalent orbitals; geometry is tetrahedral with 109.5° angles.
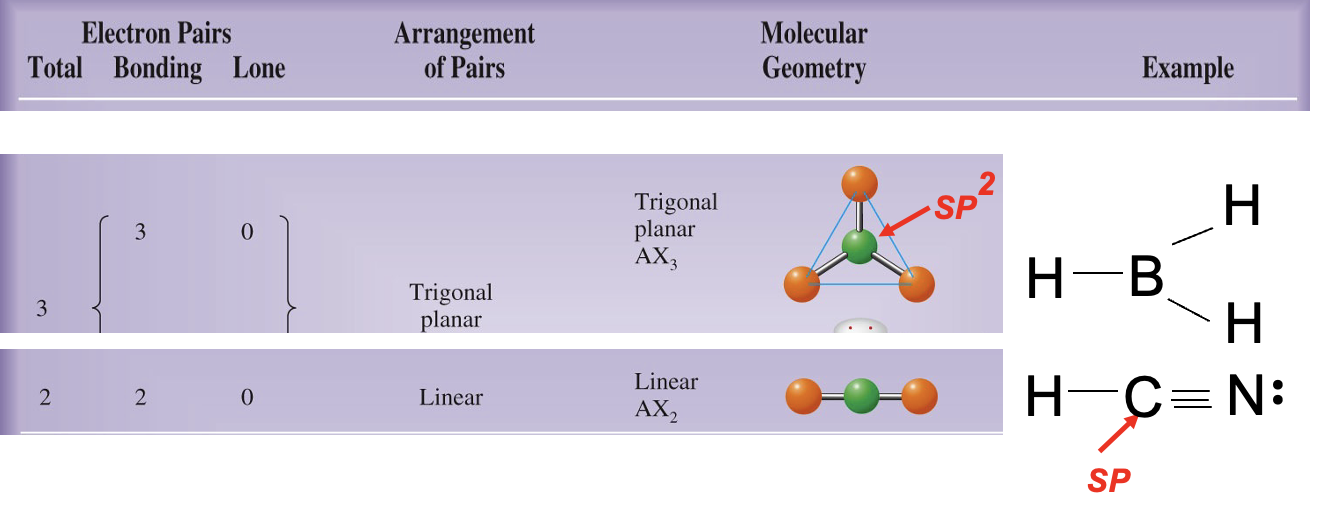
What is carbon hybridization SP2 and its geometry?
SP2 hybridization forms three orbitals in a trigonal planar arrangement (approximately 120°); a remaining unhybridized p orbital forms π bonds.
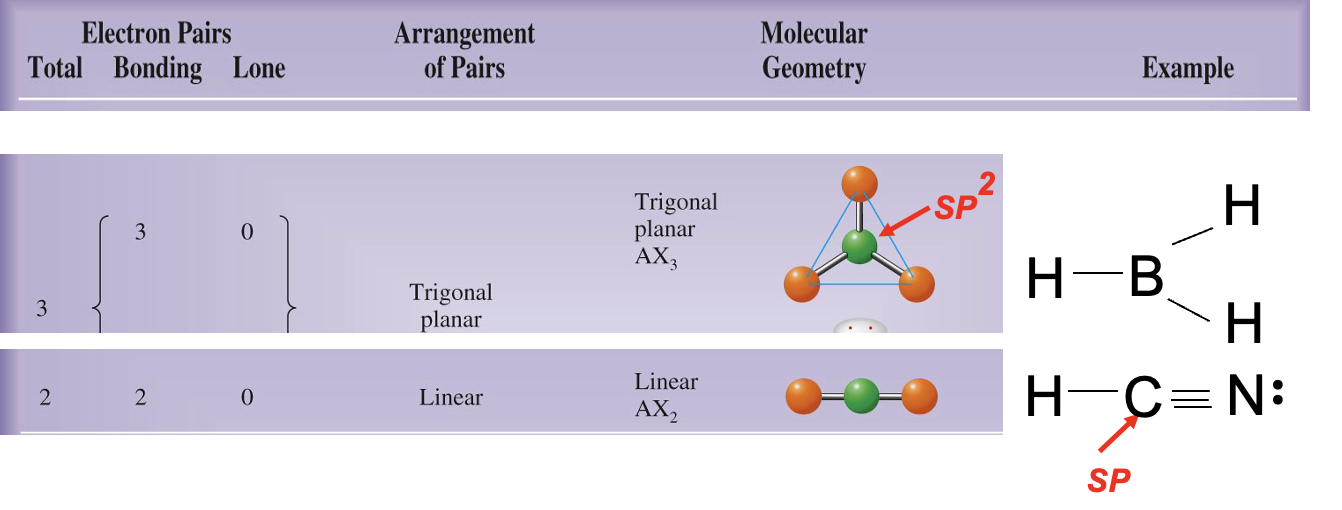
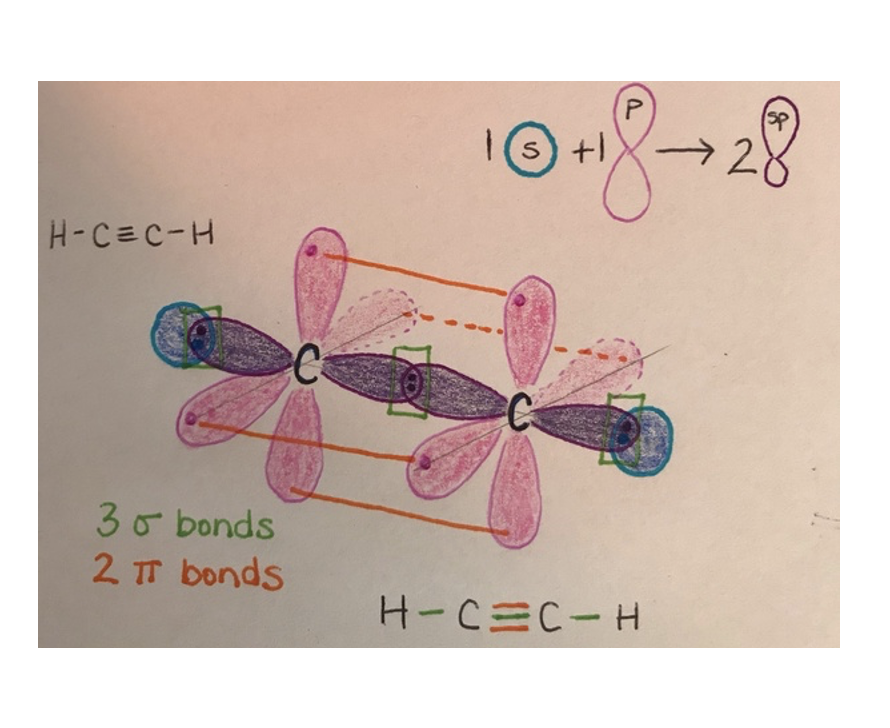
What is carbon hybridization SP and its geometry?
SP hybridization forms two orbitals in a linear arrangement (180°); two unhybridized p orbitals can participate in π bonding.
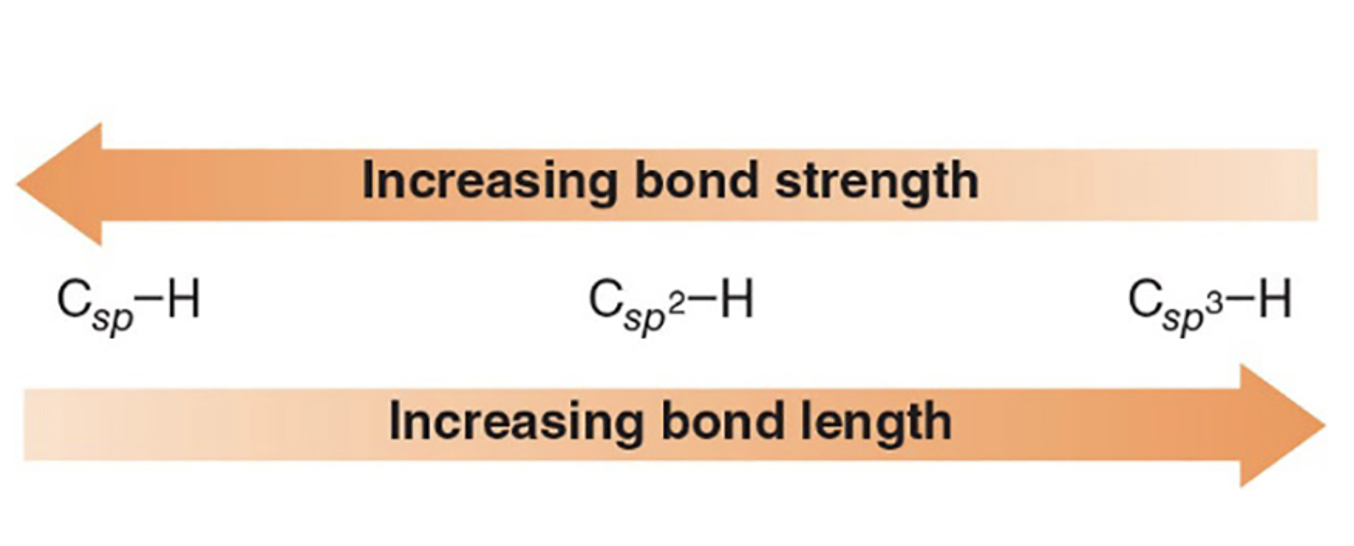
How does bond strength relate to s-character in hybrid orbitals?
Higher s-character in hybrid orbitals leads to stronger, shorter bonds; SP > SP2 > SP3 in terms of bond strength.

What is the trend in C–H bond strength with different carbon hybridizations?
C–H bonds: C–H in sp > sp2 > sp3 (more s-character yields stronger bonds with shorter length).
What is the trend in bond lengths for single, double, and triple carbon–carbon bonds?
Triple bonds are shortest and strongest, followed by double bonds, then single bonds.
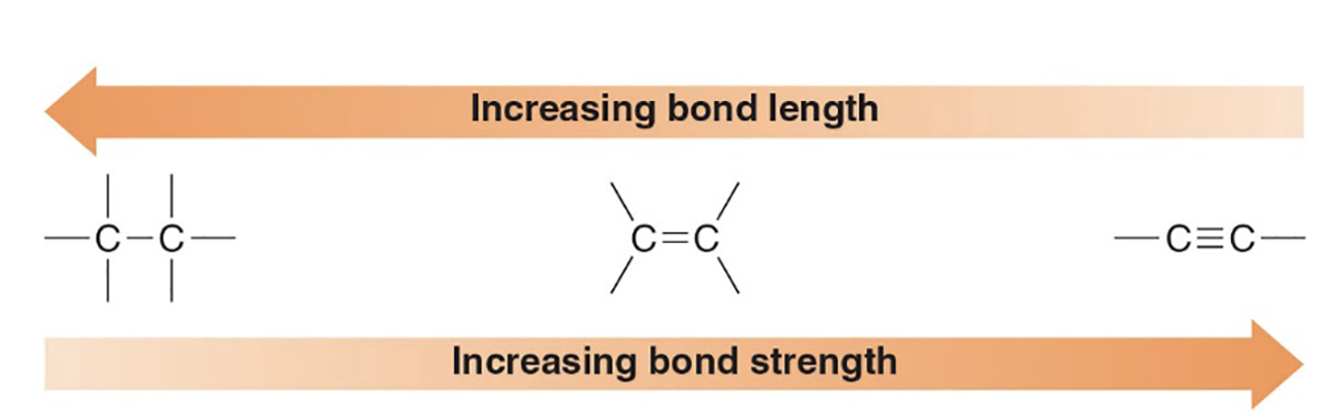
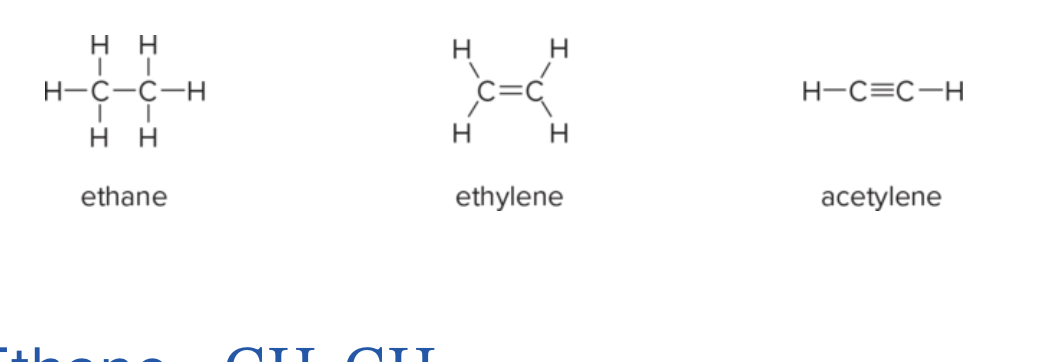
What are the typical C–C bond lengths for ethane, ethene, and acetylene (in pm)? what is their types of bonds and geometry?
Ethane (C–C in CH3–CH3): 153 pm; tetrahedral, sp3, and all sigma bonds
Ethene (C=C in CH2=CH2): 134 pm; trigonal planar, sp2, and all sigma in the same plane
Acetylene (C≡C in HC≡CH): 121 pm; linear, sp, and contains 1 sigma and 2 pi bonds
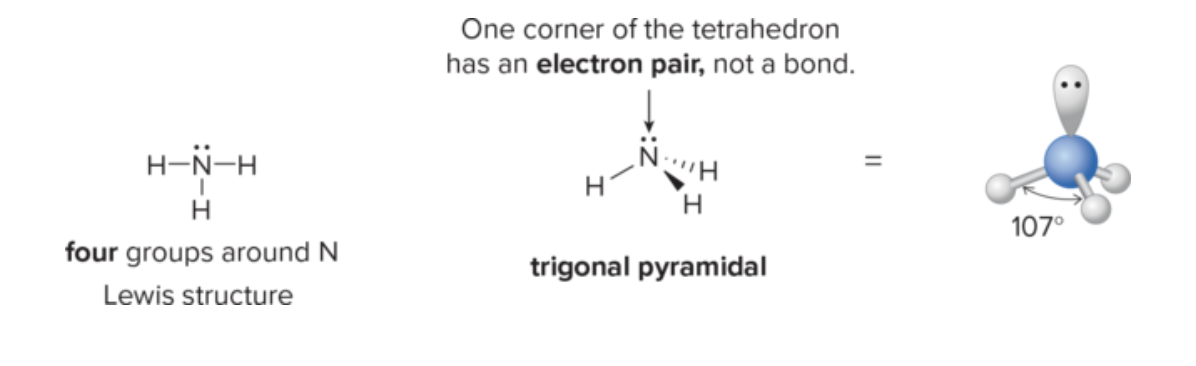
What is the relationship between molecular geometry and hybridization around a central atom?
The geometry (e.g., tetrahedral, trigonal planar, linear) is dictated by the hybridization state of the central atom and the number of bonding and lone pairs.
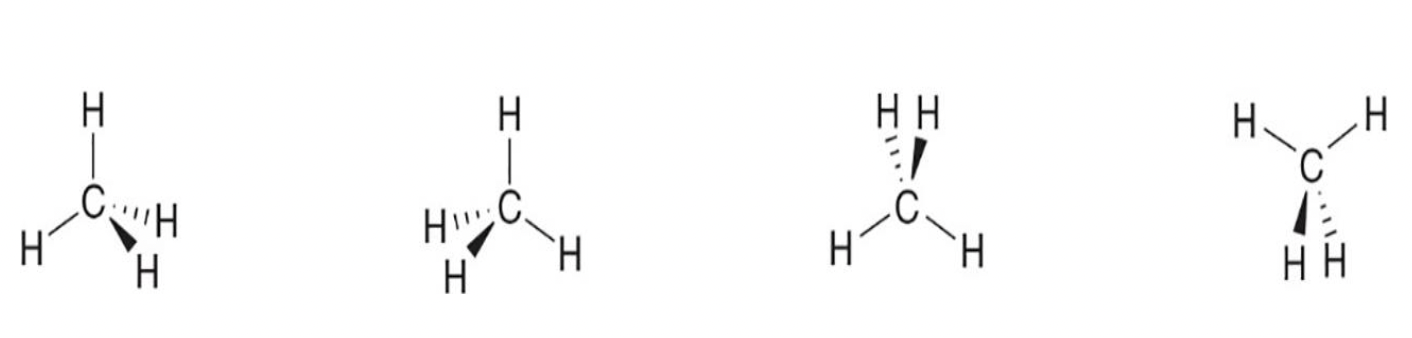
What is the 3D depiction terminology for bonds going in front of or behind a plane?
Wedges indicate bonds coming out toward the viewer; dashed wedges indicate bonds going behind the plane.
What rule helps predict a molecule’s hybridization by counting lone pairs around the central atom?
Count the number of electron domains (bonds and lone pairs); lone pairs influence the hybridization and thus the geometry.
What is the difference between polar and nonpolar molecules with examples?
Polar molecules have net dipoles due to asymmetrical bond polarity or geometry (e.g., H2O); nonpolar molecules have canceling dipoles or no polar bonds (e.g., CO2).
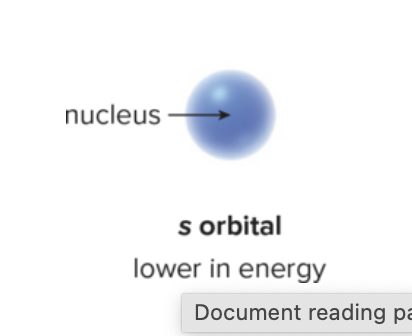
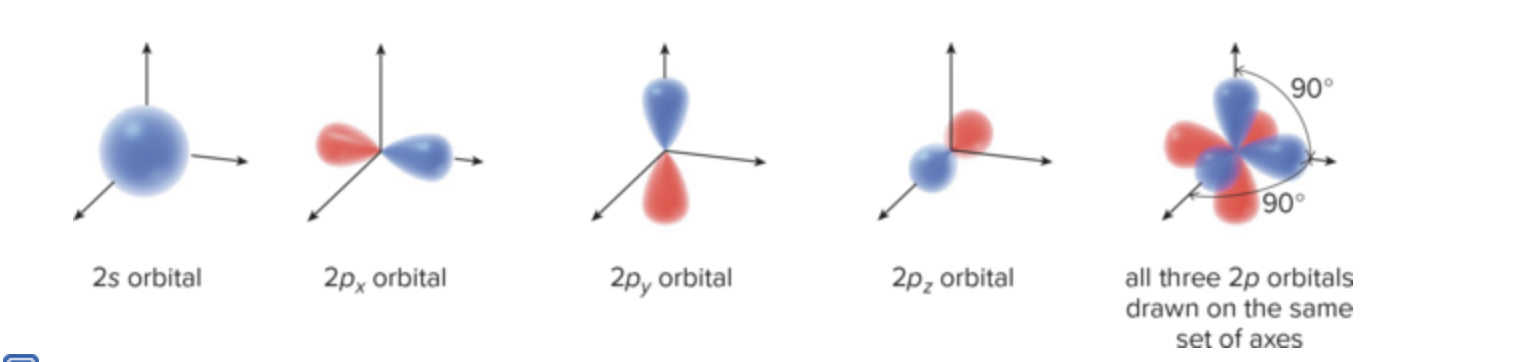
What is the s orbital? Why is it significant?
lowest sphere of electron density
lower in energy than other orbitals because electrons are kept closer to positively charged nucleus
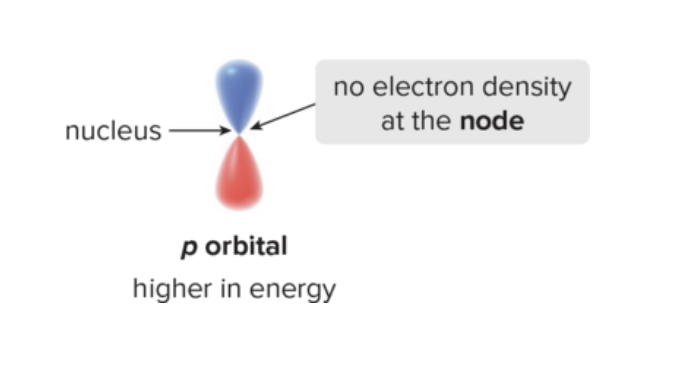
What is the p orbital? What is its shape? What does its node mean? Why is its energy higher than s (even in same shell)?
has a dumbbell shape
Node of e density at nucleus with no electron density
A p orbital is higher in energy than an s orbital (in the same shell) because its electron density is farther away from the nucleus.
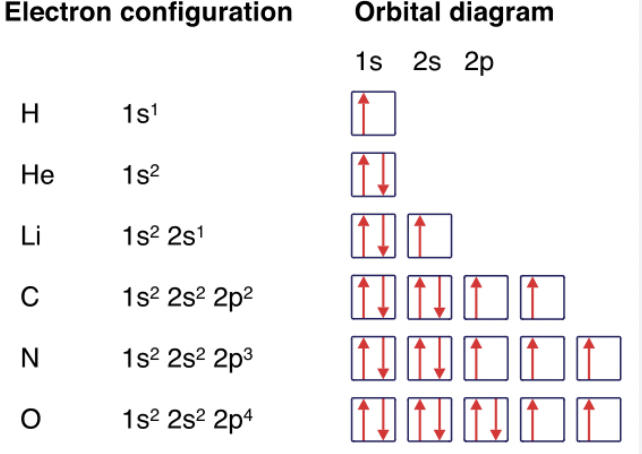
What are the rules for orbitals for 1st row elements? How many orbitals do they have? How many electrons can each orbital hold, and what does that mean?
First row elements formed by adding e- to 1st shell of orbitals around nucleus. There is only one orbital in the first shell, called the 1s orbital.
Each orbital can have a maximum of two electrons, which means only 2 elements have this.
Hydrogen (H) 1s1 configuration with one electron in 1s orbital Helium (He) has a 1s2 configuration with two electrons in 1s orbital
What are the rules for orbitals for 2nd row elements? How many orbitals do they have? How many electrons can each orbital hold, and what does that mean?
Every element in 2nd row has filled first shell, so they have 1s2
Each element in the second row of the periodic table also has four orbitals available to accept additional electrons:
1 2s orbital (s orbital in the second shell) and 3 2p orbitals, all dumbbell-shaped and perpendicular to each other along the x, y, and z axes
Because each of the 4 orbitals in the second shell can hold two electrons, there is a maximum capacity of eight electrons for elements in the second row.
The second row of the periodic table consists of eight elements, obtained by adding electrons to the 2s and three 2p orbitals.
What is bonding, and what does it aim to achieve?
Bonding is the joining of two atoms in a stable arrangement.
Through bonding, atoms aim to have a complete outer shell of valence electrons via gaining, losing, or sharing electrons.
Their goal is to be similar electron configuration to noble gas closet to them and thereby achieve stability.
How many electrons does 1st and 2nd row element want to have?
1st row: 2 (more like Helium)
2nd row: 8 (more like Neon)
How many covalent bonds does atom form for 1st and 2nd row elements?
1st row: 1 covalent bond (like Hydrogen)
2nd row: up to 4 covalent bonds (like Carbon); also consider how many valence electrons it has
Atoms with one, two, three, or four valence electrons: form one, two, three, or four bonds, respectively, in neutral molecules
Atoms with 5+: form enough bonds to give an octet.
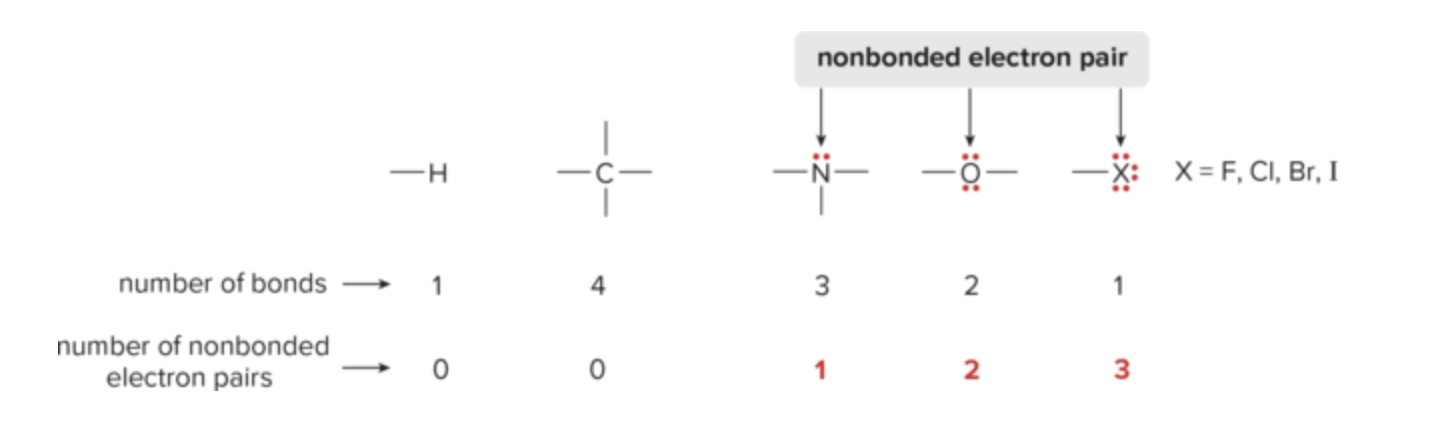
What are the rules for drawing Lewis Structures?
draw only valence e-
give every 2nd row element no more than 8 e-
give each hydrogen 2 e-
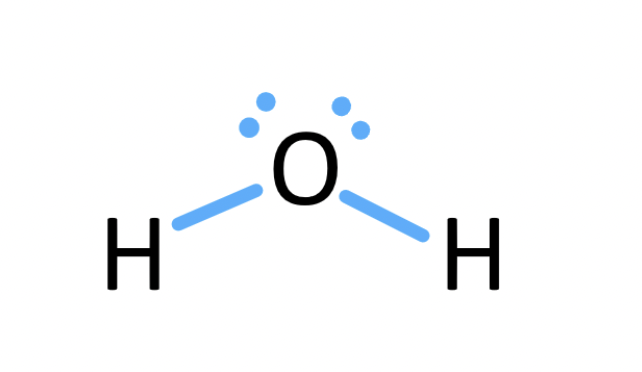
Procedure for Drawing Lewis Structures
arrange atoms next to each other you think are bonded together (ie. Hydrogen and halogens on periphery b/c only form 1 bond)
count electrons (add 1 for each - charge and subtract for +)
assign electrons around atoms (place bond btw every 2 atoms, giving 2 e- to H and no more than 8 for 2nd row element) (use remaining e- to fill octets with lone pairs or multiple bonds)
asking formal charges to all atoms
What is formal charge?
charge assigned to individual atoms; indicates how many e- around specific atom vs number of valence e-
Formula for formal charge
number of valence e- - number of e- atom owns (aka number of bonds and lone pairs)

Formula for atoms owned
number of unshared e- +1/2 x number of shared e-
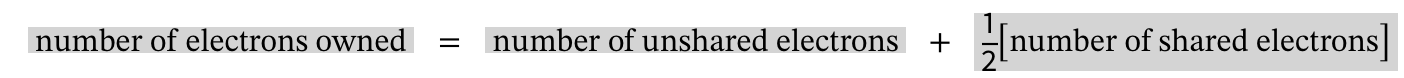
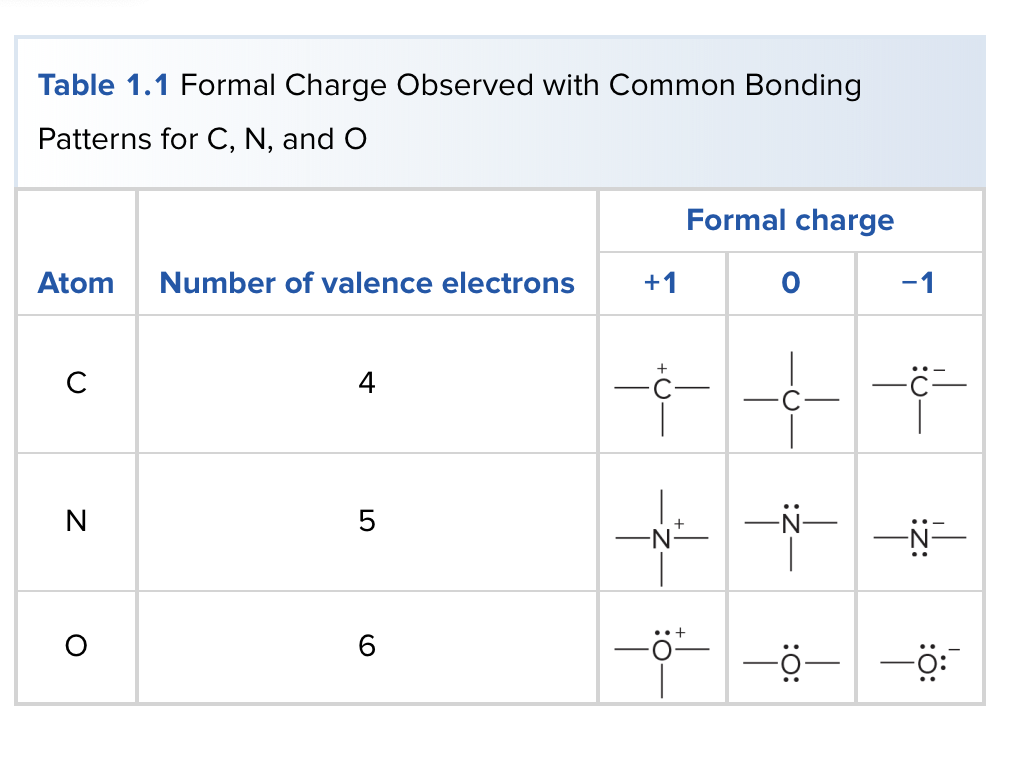
Formal Charge Table
Observed with common bonding patterns to show the formal charge on individual atoms in molecules.
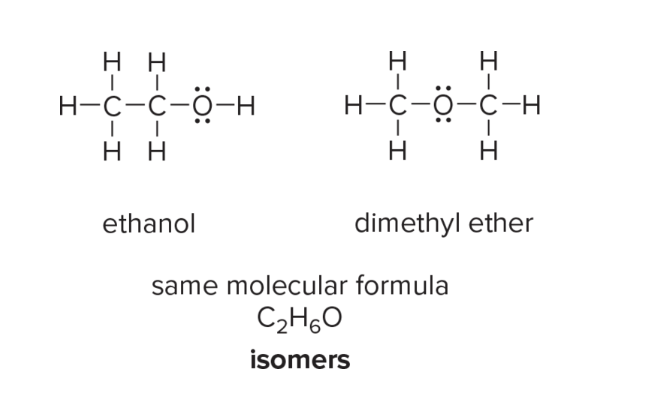
What are isomers? What are constitutional isomers and stereoisomers?
different molecules that have the same molecular formula but different structures or arrangements of atoms.
Constitutional: same molecular formula but different connectivity of atoms
Stereoisomers: same connectivity of atoms but different spatial arrangement
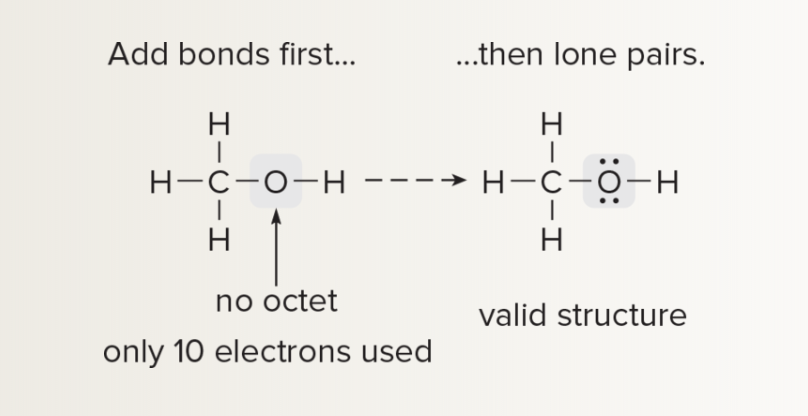
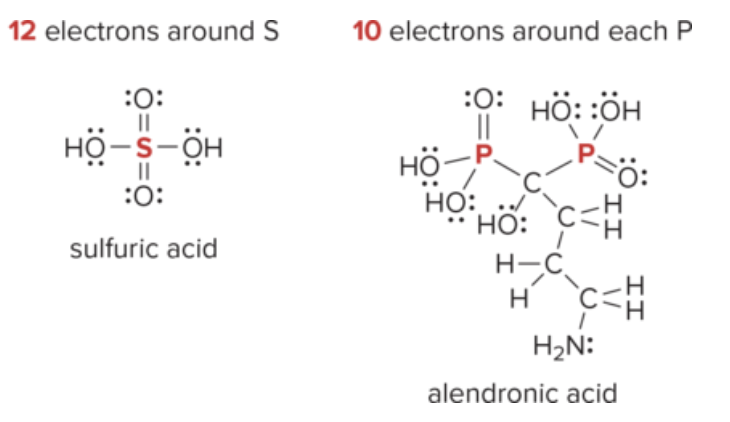
Exceptions to Octet Rule
Hydrogen: only needs 2 e-
boron and beryllium: do not have enough valence e- to form an octet in a neutral molecule
phosphorus and sulfur: may have more than eight e-
What are Resonance structures? What are they showing?
2 Lewis structures with same placement of atoms BUT different arrangement of e-
help illustrate delocalization of electrons in certain compounds.
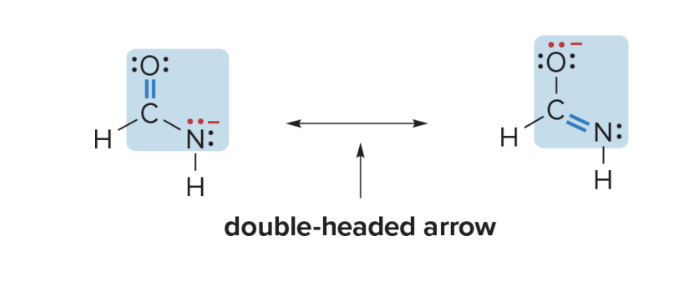
3 rules to draw resonance structures
2 R structures differ in position of multiple bonds and non-bonded e- (placement of atoms and single bonds STAY THE SAME!!)
2 R structures must have same number of unpaired e-
must be valid lewis structures
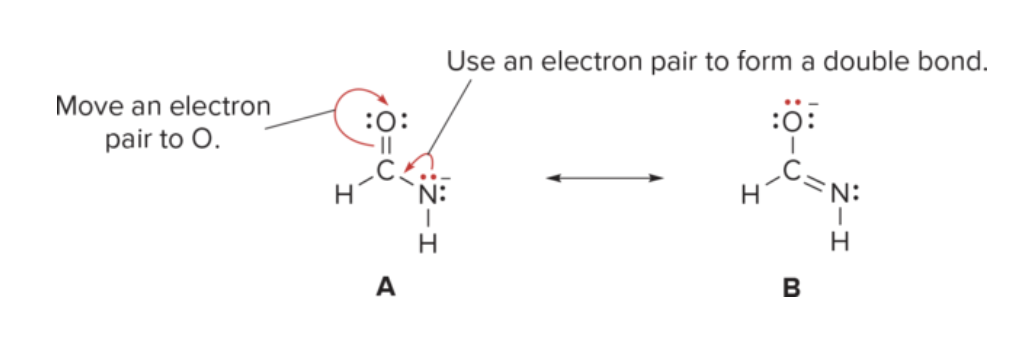
What is a resonance hybrid?
composite of all R structures that represents the actual electron distribution in a molecule, showing delocalization of electrons across certain bonds.
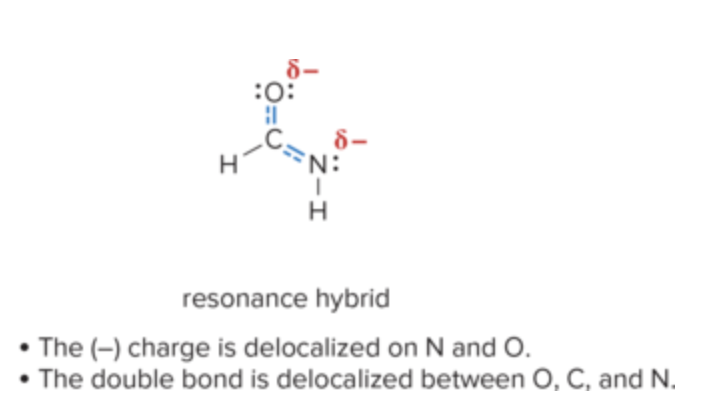
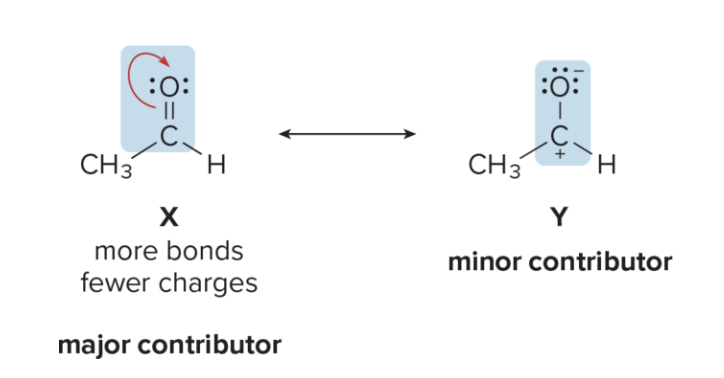
What is major contributor? What is minor contributor? how do you identify them?
Major: better R structure
Minor: valid but less stable
major contributors have lower energy and more stable e- arrangements, while minor contributors have higher energy and less favorable arrangements
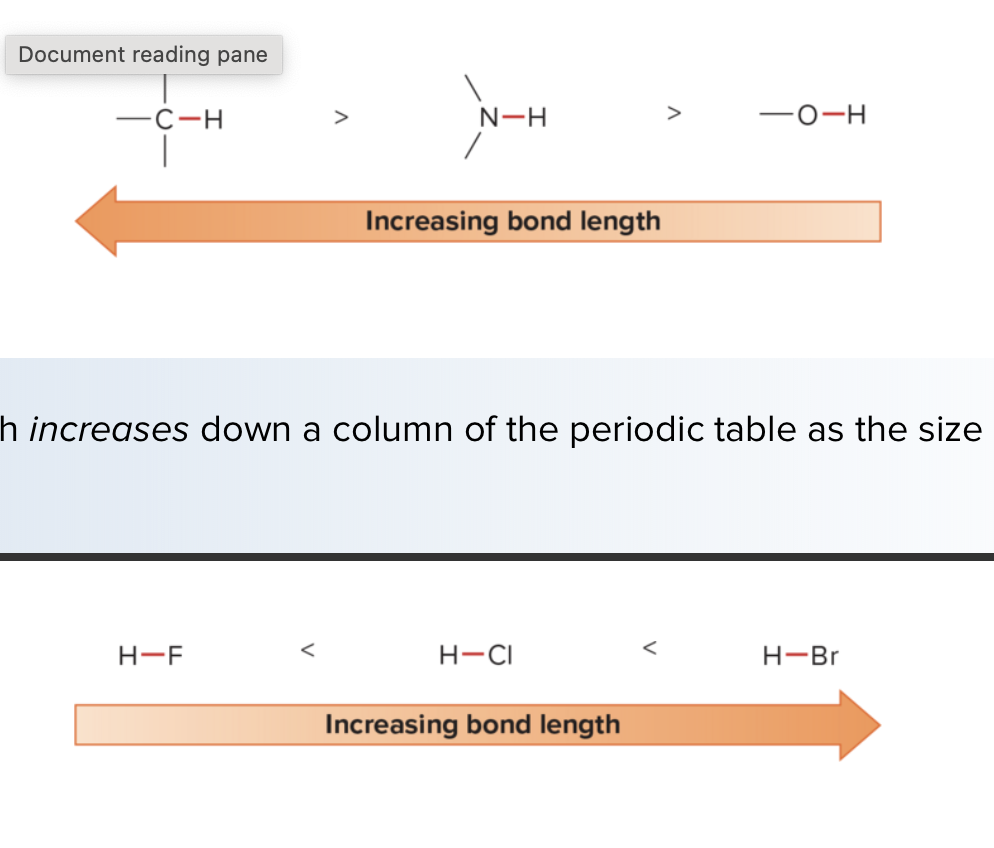
What is the trend for bond length?
Decreases across row of the periodic table as the size of the atom decreases and increases down a group as the atomic size increases.
What is bond angle?
determines shape around atom bonded to 2 other atoms
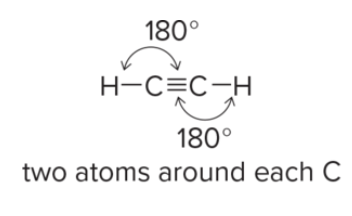
Predicting Bond Angle and geometry
Don’t forget bent with 104.5 degrees
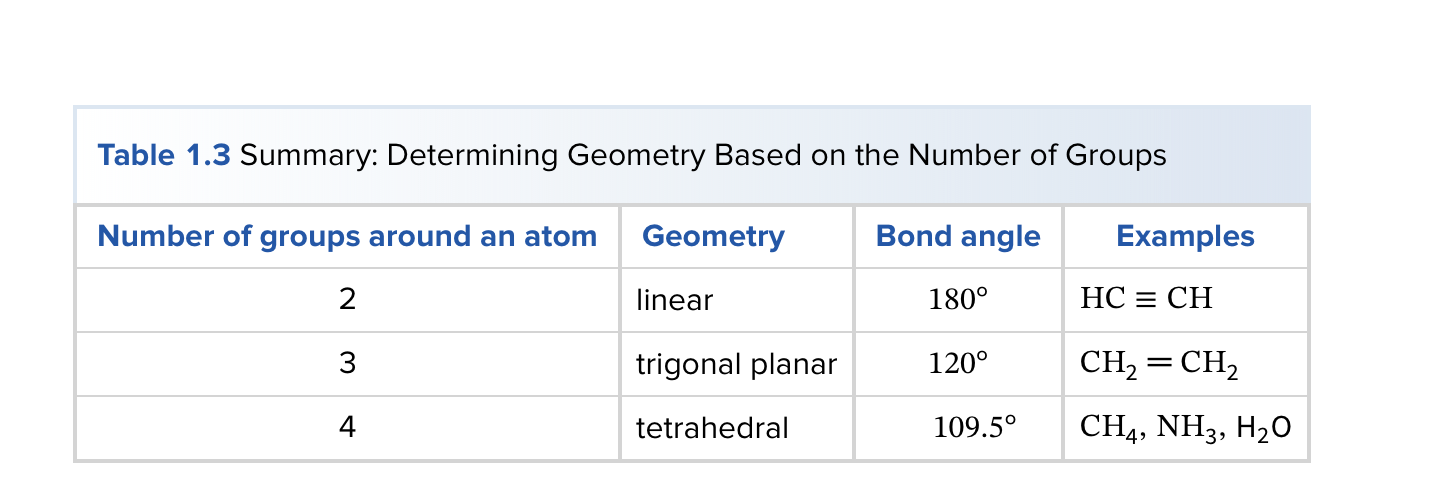
How do you predict bond angle?
count number of groups surrounding atoms
use VESPER: e- pairs repel each other to minimize repulsion and determine geometry.
How to draw skeletal structure
place C atoms as intersections of any 2 lines and end of any line
Add enough H to make C tetravalent
Add lone pairs to heteroatom to complete octets
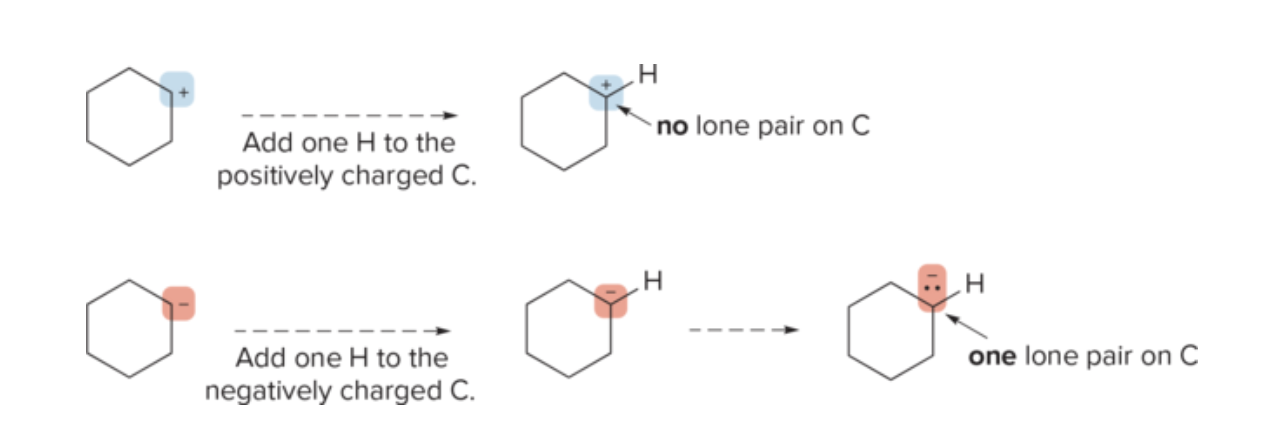
How to account for charged atoms in skeletal structures?
Indicate charge on the atom with a '+' or '-' sign.
2. Adjust the number of bonds or lone pairs to reflect the charge and octet rule.
The charge determines the number of lone pairs. Negatively charged carbon atoms have one lone pair and positively charged carbon atoms have none.
Hybridization
2 or more orbitals combine to form same number of hybrid orbitals, which leads to each having the same shape and energy
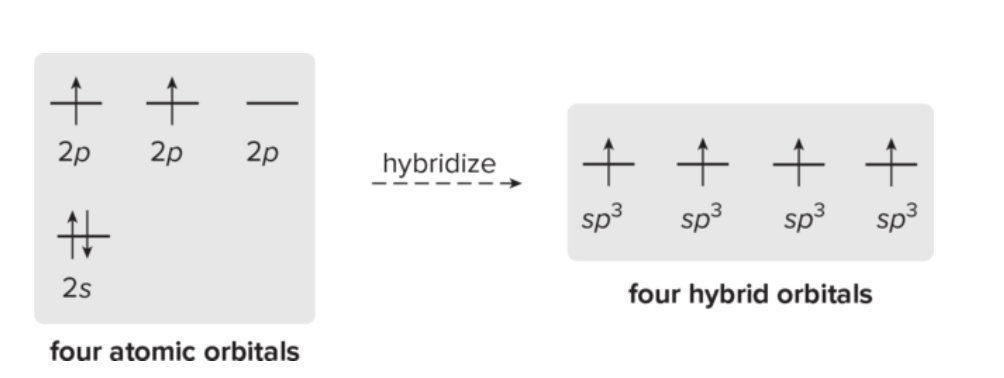
How many orbitals form each type of hybrid orbitals?
1 2s orbital and three 2p orbitals form four sp3 hybrid orbitals
1 2s orbital and two 2p orbitals form three sp2 hybrid orbitals
1 2s orbital and one 2p orbital form two sp hybrid orbitals
Difference between polar and non polar molecule
Polar: either has 1 polar bond or 2 or + bond dipoles that reinforce
Nonpolar: either no polar bonds or 2 or + bond dipoles that cancel