BM423 - Block C (toxic metals)
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
what is STEMDRL? (name + what it is + accreditation + QA)
scottish trace element and micronutrient diagnostic research laboratory
---
national service offering both analytical and clinical interpretations
---
centralised to Royal Infirmary (since to specialised/small)
---
accredited by UKAS
---
EQA by NEQAS and INSTAND
how many elements have been historically deemed essential for humans? what are some examples?
19
---
hydrogen, carbon, oxygen, selenium, nitrogen, potassium, sodium, magnesium, calcium, iodine, chlorine, phosphorous, manganese, copper, zinc, cobalt, iron, sulphur, molybdenum
how many elements are thought to be essential for humans? (i.e., not historically considered important but new evidence is suggesting)
7
---
vanadium, chromium
nickel, arsenic, silicon, tin, fluorine
---
(all in small doses)
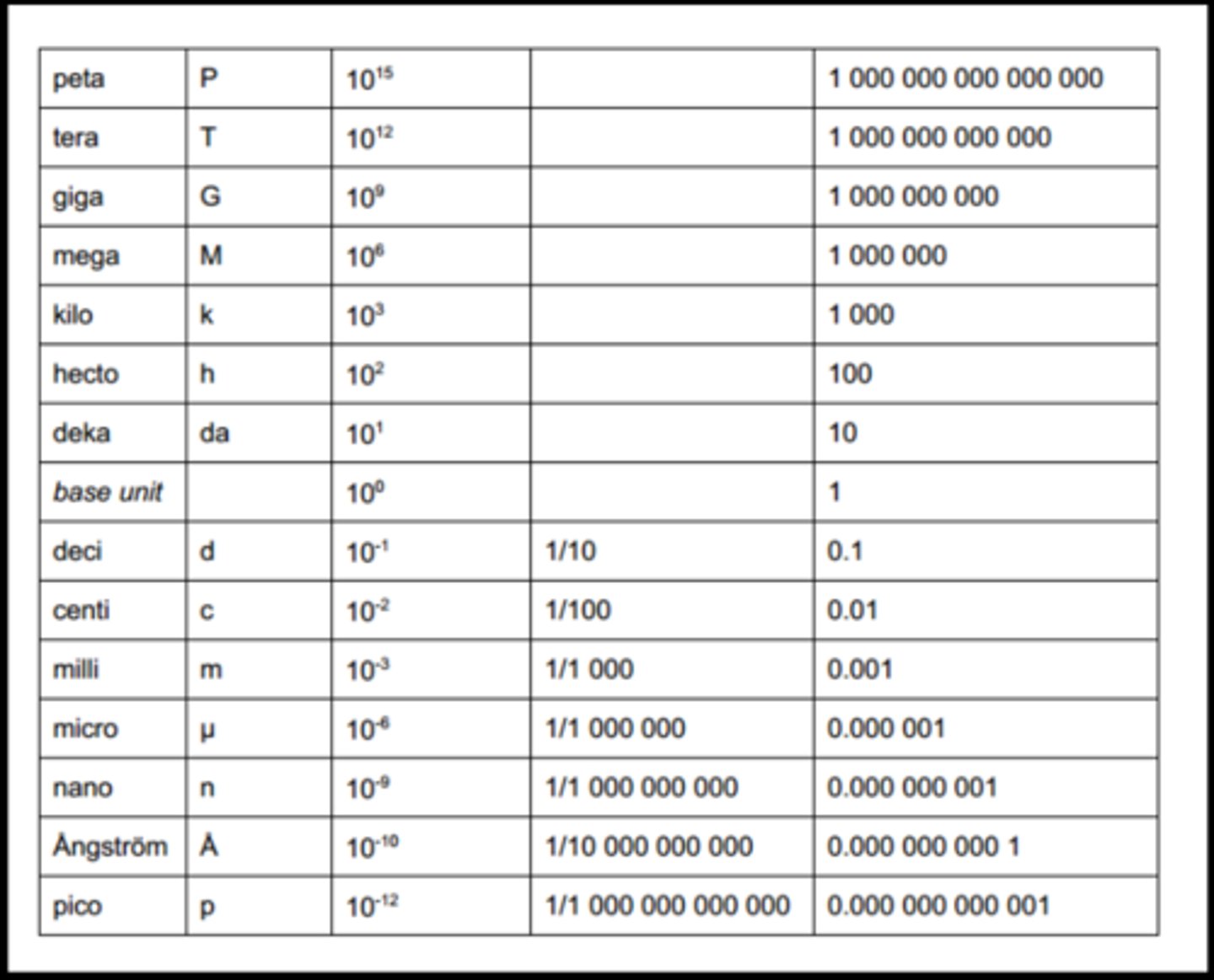
at what quantity is something considered to be "trace"? (not numerical value, units)
anything including or less than micromol/L (nanomol or picomol/L)
---
[visual of different units/volumes relative to one another]
-
with regards to essentiality, what three categories can elements be split into? is each element exclusive to a single category?
trace
= essential elements
---
ultra trace
= probably essential elements
---
toxic
= potentially toxic elements
---
no, some elements can all under both ultra trace and toxic (since they are essential at low levels but any slight increase could be toxic)
what is IUPAC?
international union of pure and applied chemistry
---
determine what is trace vs ultra trace
how does IUPAC define trace and ultra trace metals? (units)
trace = <100ppm
>>> fluid
in microg/L (<100,000)
>>> tissue
in mg/kg (<100)
in micromol/L (depends on molar mass of element)
---
ultra trace = <1ppm
>>> fluid
in ng/L (<1,000,000)
>>> tissue
in microg/kg (<1)
in nanomol/L (depends on molar mass of element)
which elements should be present in trace amounts normally? (<100ppm)
iron, zinc, copper, iodine, cobalt, selenium
which elements should be present in ultra trace amounts normally? (<1ppm)
chromium, manganese, mobyldenum, boron, vanadium, nickel, silicon, arsenic
why are essential elements like magnesium, chlorine, oxygen, etc not considered in trace and ultra trace categories?
they should not be present in trace amounts - they are required in much larger amounts (they are more essential)
what bracket/curve exists in terms of an element being required in trace amounts vs. being toxic? (hint: curve)
there is a small bracket where concentration of the element is important for biological function
---
if it is lower then the person is deficient (and could die)
---
if it is higher then the person could experience toxicity (and could die)
---
need to maintain optimum concentrations
what factors can contribute to an inviduals optimal concentration for any essential trace element?
the person's weight, genetics, or general lifestyle (health/diet)
what are the four avenues of human exposure to toxic trace elements?
occupational (welding, demolition, recycling)
---
environmental
---
accidental (whilst decorating, use of non-approved cosmetics/medicines)
---
intentional (homicide/suicide)
why might decorating expose someone to toxic trace elements?
exposure to lead paint (potentially)
---
lead
why might water (environment) expose someone to toxic trace elements?
can be public or private with private sources having less regulation (hence higher risk of contamination)
---
can be from old building (= lead pipes)
---
lead
what technique do STEMDRL use to analyse samples for trace elements? how does this work?
inductively coupled plasma mass spectrometry (ICP-MS)
---
sample is nebulised into fine mist and carried by a gas (usually argon)
>>>
mist enters "plasma torch" (= hot argon gas)
>>>
plasma torch ionises atoms in the sample
>>>
ions now enter mass spectrometer
>>>
etc
---
good technique when you want to measure small amounts/trace amounts
why is trace element analysis centralised?
ICP-MS is expensive and technically demanding
what turnaround is associated with ICP-MS/trace element analysis?
can vary depending on sample type - usually 1-3 days but if sample requires a lot of processing (e.g., if it is a solid sample) could take up to a week
what are some common issues with ICP-MS? (2)
- contamination (hard to keep environment sterile)
---
- falsely low results (common with lead - it tends to hide inside RBCs so if plasma is analysed, results are falsely low)
summarise the role of argon in ICP-MS?
sample nebulised > carried by argon gas to plasma torch
>>>
(prevents contamination via elements in sample interacting with other gases - only weakly interacts with argon hence why it can carry but not contaminate)
---
plasma is hot argon
>>>
collides with elements in the sample to ionise them into positively charged ions to allow them to be analysed
ICP-MS most often uses a quadropole mass filter to analyse ions; what does this involve?
quadrupole filter
=
two rods connected to RF voltage and two rods connected to DC voltage
=
creates oscillating square electric field
---
frequency of filter is set to one that will only allow ions of a specific m/z to pass through
>>>
amount of ion detected is proportional to amount of that element present
>>>
quadrupole sequentially adjusted to account for other ions m/z and allow full sample analysis
what happens inbetween ionisation and MS/quadropole filter interaction in ICP-MS?
ions enter the collision cell where the sample is purified of polyatomic ions via hydrogen interaction
---
(i.e., remove a big molecule that could be confused for a heavy ion)
---
bigger molecules = bigger target for H+/H2 = more likely to be broken up in collision cell
STEMDRL offers testing on 4 sample types: what are they?
whole blood (unclotted)
---
urine
---
plasma
---
hair
what toxic trace elements would be assessed from a whole blood sample? (6)
lead
---
mercury
---
cadmium
---
manganese
---
chromium
---
cobalt
what toxic trace elements would be assessed from a plasma sample? (1)
aluminium
what toxic trace elements would be assessed from a urine sample? (6)
cobalt
---
chromium
---
cadmium
---
mercury
---
lead (sometimes)
---
arsenic
what toxic trace elements would be assessed from a hair sample? (2)
mercury
---
arsenic
in summary, what patient samples would be used if we are looking for arsenic?
hair or urine
in summary, what patient samples would be used if we are looking for lead?
whole blood (or urine if exposure recent/acute enough)
what is a common source of chromium/cobalt in the blood?
components of joint replacements
---
overtime rubbing/frictions results of release of the metals into the blood
why would a seafood loving patient pose a problem for STEMDRL?
seafood contains arsenic
---
can obtain a falsely high result if consumed within 5 days of testing
---
further testing can be done to distinguish between true poisoning and dietary arsenic (different chemical forms) (methylated tends to indicate it will be excreted)
why would a patient with Wilson's Disease pose a problem for STEMDRL? (2)
= inherited disorder of copper metabolism
---
leads to accumulation of copper in various tissues
---
would be present in large excess (as opposed to in trace amounts)
---
would throw off ICP-MS (since it is not calibrated to cope with large amounts - only trace)
>>> impacts readings of other ions
---
ALSO
---
normally excess copper will be stored in tissues and/or excreted via bile so may not even show as excess in a trace metal analysis
what are the most common trace metal exposures?
arsenic, mercury, and lead
---
exposure not so common anymore because we have better health and safety/regulations
in terms of lead, why has exposure incidence decreased in the last 50 years? (3)
shift to unleaded petrol (1980s)
---
ban of lead paint (1990s)
---
water treatment for lead pipes (late 1970s)
how is water treated for lead? (2)
increasing the pH of the water source (e.g., loch) to reduce acidity and, hence, prevent leaching lead from the solder
---
addition of phosphates to provide protective coating around lead pipes
why is it difficult to evaluate the prevalence of various toxic elements in the environment?
they are not routinely measured - only in instances of toxicity (so we can't really see normal/baseline/reference amounts)
air, soil, and water are the three environmental sources of toxic elements? what contaminates these sources? how do these sources interact with humans?
air:
industrial/auto/natural emissions
>>>
inhaled as air or dust, contaminates soil and water
---
soil:
crustal weathering or waste water discharge
>>>
inhaled as dust, contaminates food sources, directly ingested by human
---
water:
water waste discharge or plumbing
>>>
contaminates food, contaminates drinking water
what is important to establish once you have determined a patient has been exposed to a toxic element?
the source of exposure
what factors contribute to the risk of toxicity following lead exposure?
age, physiological status, nutritional status, genetics
---
not everyone will be affected in the same way (two people who exposed to same amount - one might be fine, one might get sick)
why is lead so toxic? (hint: what does it mimic?)
it is similarly sized to calcium
---
often competes with calcium
---
(e.g.,) absorption in GI > competes with calcium
in what sense can age impact lead exposure/toxicity?
children have growing bones
>>>
the body cannot discriminate well between calcium and lead
>>>
lead is mistakenly incorporated into the growing bone
>>>
over time, the locked-in lead slowly leaches into the blood
what is the main reason for lead toxicity/lead exhibiting toxic effects?
its similarity to calcium
---
affects any bodily process involving calcium (e.g., interferes with calcium signalling > affects blood pressure/heart rate/muscle contraction)
what is the main route of lead exposure?
general public = ingestion
>>>
5-15% of ingested lead is absorbed by GI (can be up to 45% in fasting conditions given increased motility of GI and lack of calcium)
(can be up to 53% in children)
---
occupational = inhalation
(deposits 30-50% of inhaled lead in the lungs)
where does ingested/absorbed lead tend to deposit within the body?
distributed by blood to soft tissues (liver, kidney, brain, etc) and bones (i.e., where calcium is most needed)
what half-life is associated with lead in bone and soft tissue? why does this differ? (2)
soft tissue/blood = 20-40 days
---
bone = 10-30 years
---
in bone, lead deposits in an insoluble form (lead phosphate)
---
lead deposits in areas of increased bone growth, becoming incorporated into the bone matrix (hence protecting it/preventing excretion)
what routes of excretion are associated with lead?
primarily excreted in urine
---
1/3 excreted in faeces
---
small proportion excreted via sweat, saliva, hair/nails, breast milk
does route of exposure determine toxicology of lead (i.e., inhalation vs ingestion)?
no - toxilogical effects same regardless of exposure
urine is the primary excretion route for lead, yet to assess lead we measure whole blood: why?
levels in urine are associated with excretion - this could be a lag between active exposure and past exposure
---
blood shows what is currently being circulated in the body hence would more accurately reflect current/active/acute lead exposure
a patient ingests 1000 atoms of lead - what distribution do you expect in RBCs? plasma? bone/soft tissue?
99% of the ingested amount will be stored in RBCs (inactive)
= "990" in RBCs
---
1% remains free/active in the plasma
= "10"
---
of the 1%, 90% is absorbed by the bone/skeleton
= "9"
---
of the 1%, the remaining 10% is absorbed by soft tissue
= "1"
to what RBC functional groups does lead preferentially bind? (3)
HbA
---
SH (sulfhydryl) (antioxidant groups)
---
ALAD (enzyme within RBCs) (inhibited by lead - decreased activity indicator of lead toxicity) (involved in haem synthesis)
what are the three major mechanisms of lead toxicity?
binding to sulfhydryl groups on proteins + inhibiting enzymes (such as ALAD)
>>>
inhibition/denaturation of enzymes and structural proteins
---
substitutes/mimics other divalent cations (zinc + calcium)
---
source of free radicals (promotes oxidative stress by inhibiting anti-oxidants (e.g., it binds glutathione/inhibits SOD))
what are the signs/symptoms of acute lead toxicity?
blood lead level (microg/dL)
>>> symptom
---
40-60
>>> GI symptoms (children)
---
40-80
>>> acute interstitial nephritis
---
48-120
>>> hypertension
---
80-100
>>> encephalopathy (children)
---
100-120
>>> encephalopathy (adults)
---
100-400
>>> GI symptoms (adults)
what are the effects of acute lead toxicity on the kidney(s)?
proximal renal tubular dysfunction
>>> phosphaturia, aminoaciduria, glycosuria, renal tubular acidosis
---
(impaired bulk nutrient reabsorption given proximal dysfunction)
what are the two major signs/symptoms of acute lead toxicity?
encephalopathy (headache, confusion, drowsiness)
---
GI symptoms (cramping, nausea, vomitting, constipation)
which is more common, acute or chronic lead toxicity? why?
chronic
---
acute would require significant exposure in a short period of time
what are the haematological signs/symptoms of chronic lead toxicity?
anaemia
>>>
lead inhibits...
...ALAD (delta-aminolevulinic acid dehydratase)
+
...ferrochelatase
+
...coproporphyrinogen oxidase
---
basophilic stippling and reticulocytosis (=both reflective of impaired erythrocyte development/RNA presence in circulating RBC - lead inhibits enzymes involved in erythropoiesis)
summarise the development/synthesis of haem? what parts of this development can be interupted by lead? (3)
succinyl CoA + glycine
>>>
delta-aminolevulnic acid (ALA)
>>> [ALAD]
porphobilinogen
>>>
hydroxymethybilane
>>>
uroporphyrinogen I and II
>>>
(...II) uroporphyrin III and coproporphyrinogen III
>>> [coproporphyrinogen oxidase]
(copro...) protophyrinogen IX
>>>
protoporphyrin IX
>>> [ferrochelatase]
haem
---
ALA > porphobilinogen (ALAD inhibition)
coproporphyrinogen III > protophyrinogen IX (coproporphyrinogen oxidase inhibition)
protoporphyrin IX > haem (ferrochelatase inhibition)
what are the hepatotoxic effects of chronic lead toxicity? (hint: paracetamol poisoning aligned)
reduced haem synthesis
>>>
reduced cytochrome p450 activity (essential for drug metabolism, among other things (includes haem as functional component))
what are the neurotoxic effects of chronic lead toxicity?
reduced nerve conduction
---
postural sway
---
tremor
---
intelligence/neurobehavioural effects
---
all due to impacted neuron/calcium signalling and/or cell damage via apoptosis
what association has been established between blood lead levels and IQ?
the higher the blood lead levels, the lower the IQ
---
primarily due to neurotoxic effect of lead (impacting signalling and/or direct damage via cellular injury response)
is lead carcinogenic?
yes (inorganic/unmethylated forms)
what effects can chronic lead toxicity have on reproductive health? (men and women)
men
= reduced semen volume/sperm counts
= decreased sperm motility
= increased sperm dysplasia
---
women
= impaired hormone synthesis
>>> alters menstruation and reduces fertility
---
pregnancy
= risk of spontaneous abortion
= adverse neurodevelopment of foetus
in summary, what are the signs and symptoms of chronic lead toxicity? (4 main)
anaemia
>>> impaired erythropoiesis via haem-synthesis enzyme inhibition (ALAD, ferrochelatase, coproporphyrinogen oxidase)
(binds to sulfhydryl groups in these enzymes > denatures/inhibits them)
---
reduced haem synthesis (via above enzyme inhibition) >>> reduced cytochrome p450 (hepatotoxic effects)
---
impaired neuronal signalling (interferes with calcium signalling + damages cells via initiating cellular injury/stress responses)
>>>
effects neurodevelopment of children particularly
---
can reduce count/mobility of sperm, hormone synthesis, and neurodevelopment of a foetus
what two laboratory assessments are carried out to assess lead?
EDTA whole blood (EDTA = anti-coagulant)
>>> reflects intake of previous 3-5 weeks
---
urine (tetraethyl lead)
>>> can reflect a recent exposure
(if body has not had time to metabolise it > excreted in large amounts in urine)
(low blood but high urine lead)
---
tetraethyl lead was in petrol prior to unleaded petrol
---
may be some industrial exposure as well
what is the clinical reference range for a safe blood lead concentration?
there is no official safe concentration but...
---
adults
= <0.1 micromol/L
---
children
= <0.1 micromol/L
what is the reference range for safe water lead concentration?
<10 microg/L
what are the two levels considered when it comes to control of lead at work?
action level =concentration/level at which specific actions must be taken to protect workers
---
suspension level
=concentration/level at which the worker(s) must be removed from the work
what is the action level of BLL? (general, fertile women, and under 18s)
general
= 50 microg/100mL
---
fertile women
= 25 microg/100mL
---
under 18s
= 40 microg/100mL
what is the suspension level of BLL? (general, fertile women, and under 18s)
general
= 60 microg/100mL
---
fertile women
= 30 microg/100mL
---
under 18s
= 50 microg/100mL
what treatment is often used in instances of high blood lead level?
chelation
---
use a chelating agent that will bind lead and supplement patient with calcium/zinc (since these will likely also be chelated)
in summary, how are trace elements measured?
ICP-MS
in summary, what are the main routes of toxic element exposure?
occupation, environmental, accidental, cosmetic
in summary, what is true of the early symptoms of lead poisoning?
non specific
in summary, what is true of lead excretion levels?
low
in summary, what does the form of lead (i.e., organic/inorganic) impact?
the symptoms