Lecture 18 Sterile Product Regulations USP <797> and <800>
1/34
Earn XP
Description and Tags
Objectives: Define the purpose of USP <797> and what products have to follow the guidelines •Know the different training and qualification requirements •Know how often a PEC should be cleaned and disinfected •Know the difference between category 1, 2, and 3 CSP’s •Know how to determine a product’s BUD •Know the additional requirements contained in USP <800>
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
35 Terms
Match each USP (United States Pharmacopial Convention) to the right kind of compounding/drug:
USP <795>
a. non-sterile compounding
b. general sterile compounding
c. hazardous drugs
a. non-sterile compounding
Match each USP (United States Pharmacopial Convention) to the right kind of compounding/drug:
USP <797>
a. non-sterile compounding
b. general sterile compounding
c. hazardous drugs
b. general sterile compounding
the MINIMUM standards to be followed when preparing a sterile compound —> standards beyond the <797> must be defined by the facility
Match each USP (United States Pharmacopial Convention) to the right kind of compounding/drug:
USP <800>
a. non-sterile compounding
b. general sterile compounding
c. hazardous drugs
c. hazardous drugs
True or false: Non-hazardous compounded sterile preparations (CSPs) administered within 4 hrs, proprietary bag & vial systems, allergenic extracts must follow <797>.
False! The do not have to follow <797>, but still must follow proper aseptic technique
Select all of the following needed to be removed for compounding:
a. all cosmetics (makeup)
b. outer garments
c. hand, write, body jewelry that interferes with PPE
d. all electronic devices
e. nail polish/artificial nails
f. all of the above
f. all of the above
Which of the following is NOT a Facility Design and Environmental Control?
a. Temperature of the room is less than 68o F and 60% relative humidity
b. Ante-room must have a line of demarcation that separates the clean and dirty side.
c. Tacky surfaces inside the ante/clean room to clean shoes
d. Hands free doors
c. Tacky surfaces inside the ante/clean room to clean shoes
FALSE: Tacky surfaces must be OUTSIDE the ante/clean room
True or false: Each facility must have a written testing and hands on proficiency training program for sterile compounding training
True! At least every 12 months personnel must prove proper sterile compounding techniques.
True or false: Brushes and hand air dryers are allowed during hand hygiene procedures.
False! They are not allowed.
How is airflow measured in maintaining sterile compounding conditions?
It is measured by the number of air changes per hour (ACPH)
Minimum requirements:
ISO class 8 > 20 ACPH
ISO class 7 > 30 ACPH
How long does recertification take for ACPH (Air Flow testing)?
a. Every 12 months
b. Every 8 months
c. Every 6 months
d. Every 2 years
c. Every 6 months
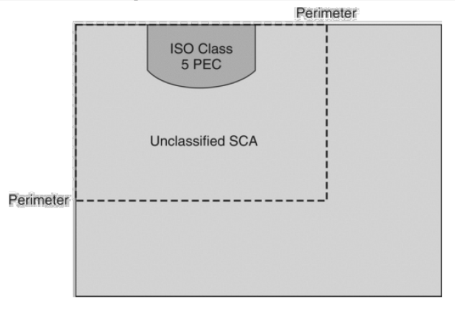
A segregated compounding area (SCA) is an area not contained in a ___ room where ___ compounding is performed. What ISO class must the PEC be used for?
buffer, sterile
ISO class: 5
Which is TRUE about the Segregated Compounding Area (SCA)?
a. ISO class 8 is used for PEC
b. Contains doors that open to the outside
c. An area within 1 meter (3.3ft) of PEC is used for sterile compounding
d. De-garbing can be done on the dirty side of SCA
c. An area within 1 meter (3.3ft) of PEC is used for sterile compounding
Match the type of cleaning agent to its definition!
Cleaning
a. Removal of residue (sterile water with surfactants)
b. Kills fungi, viruses, and bacteria (70% isopropyl alcohol, diluted bleach, hydrogen peroxide)
c. Kills fungal and bacterial spores (PeridoxRTU, SporGon, Spor-Klenz)
a. Removal of residue (sterile water with surfactants)
Match the type of cleaning agent to its definition!
Disinfecting
a. Removal of residue (sterile water with surfactants)
b. Kills fungi, viruses, and bacteria (70% isopropyl alcohol, diluted bleach, hydrogen peroxide)
c. Kills fungal and bacterial spores (PeridoxRTU, SporGon, Spor-Klenz)
b. Kills fungi, viruses, and bacteria (70% isopropyl alcohol, diluted bleach, hydrogen peroxide)
Match the type of cleaning agent to its definition!
Sporicidal
a. Removal of residue (sterile water with surfactants)
b. Kills fungi, viruses, and bacteria (70% isopropyl alcohol, diluted bleach, hydrogen peroxide)
c. Kills fungal and bacterial spores (PeridoxRTU, SporGon, Spor-Klenz)
c. Kills fungal and bacterial spores (PeridoxRTU, SporGon, Spor-Klenz)
Which is the correct cleaning order for room cleaning?
a. Clean room —> ante room —> general supply/storage area
b. General supply/storage area —> ante room —> clean room
c. Ante room —> Clean room —> general supply/storage area
d. Ante room —> general supply/storage area —> clean room
a. Clean room —> ante room —> general supply/storage area
cleaning goes from cleanest to dirtiest area
How often do you clean and disinfect the PEC and work surfaces around it?
a. Daily (when compounding)
b. Weekly
c. Monthly
a. Daily (when compounding)
How often do you use sporicidals to clean the internal surfaces of the PEC?
a. Daily (when compounding)
b. Weekly
c. Monthly
b. Weekly —> for Category 3 CSP
c. Monthly —> for category 1 and 2 CSP’s
How often do you clean, disinfect, and use sporicidals for walls, doors, ceilings, storage shelves, and equipment outside of PECs?
a. Daily (when compounding)
b. Weekly
c. Monthly
c. Monthly
True or False: Beyond Use Dates is the same as Expiration dates.
FALSE!
Expiration date is the date which the product is still able to be used under predefined conditions (manufacture guarantees the potency and safety of medication)
BUD: date and time where it is safe to use a compounded medication (calculated from the time compounding is initiated)
BUD involves a specific compounding date bc it is made for a specific prescription; Expiration date is for more standard medications (manufacturers have predefined conditions)
What do the categories correlate to in sterile compounding?
They correlate to the risk of microbial, physical, or chemical contamination.
They are risk levels that are used to determine BUD
Match each Category to its condition:
A gown can be reused by the same person in the same shift if it is kept in the anteroom. Air sampling is tested every 6 months, garbing and hand hygiene/media fill is tested every 6 months, and surface sample is tested monthly.
a. Category 1
b. Category 2
c. Category 3
d. Both a and b
d. Both a and b
Match each Category to its condition:
Sterility testing is not required
CSP is made in an SCA (Segregated Compounding Area)
a. Category 1
b. Category 2
c. Category 3
d. Both a and b
a. Category 1
Match each Category to its condition:
Sterility testing required for certain BUDs
CSP is made in a cleanroom suite
a. Category 1
b. Category 2
c. Category 3
d. Both a and b
b. Category 2
Match each Category to its condition:
No expose skin in the buffer room, a low-lint garb must be sterile (single use).
Air sampling (monthly), surface sampling (weekly), garbing/hand hygiene/media fill (q 3 months)
Sterility testing required for all products
a. Category 1
b. Category 2
c. Category 3
d. Both a and b
c. Category 3
True or false: If 1 or more components are sterile, product must be sterilized.
True! Must occur when placing product into final dispensing container
For single dose vials, when it is punctured outside of ISO class 5 air
a. use within 4 hours
b. use within 12 hours
c. use immediately
a. use within 4 hours
For single dose vials, when it is punctured inside of ISO class 5 PEC
a. use within 4 hours
b. use within 12 hours
c. use immediately
b. use within 12 hours
For single dose vials, when opening an ampule, you must..
a. use within 4 hours
b. use within 12 hours
c. use immediately
c. use immediately
When was the USP <800> Hazardous drugs enforced and what does it cover?
November 2023
Covers storage, handling, compounding, dispensing of all sterile and non-sterile hazardous drugs
Which is FALSE in a sterile compounding cleanroom suite?
a. HVAC is vented externally
b. Negative pressure to adjacent rooms
c. At least 12 ACPH
d. ISO Class 7 clean room and ante room
c. At least 12 ACPH
FALSE —> at least 30 ACPH
Which is FALSE in a sterile compounding segregated compounding area?
a. HVAC is vented externally
b. Negative pressure to adjacent rooms
c. At least 30 ACPH
d. ISO Class 7 clean room and ante room
d. ISO Class 7 clean room and ante room
When handling Hazardous Drugs (USP <800>), you must follow <797> guidelines and what?
a. an additional pair of gloves and shoe covers
b. gowns are impermeable
c. outer gloves are discarded in the PEC and placed in a hazardous garbage container
d. all garments (outer and inner) is removed in the ante room
a. an additional pair of gloves and shoe covers
b. gowns are impermeable
c. outer gloves are discarded in the PEC and placed in a hazardous garbage container
outer garments must be removed in the CLEAN room
When is Closed System Transfer Devices used (CSTD) or recommended?
Recommended during compounding of antineoplastics HDs
Required during administration