1.3 Properties of Transition Metals
1/23
Earn XP
Description and Tags
These flashcards are for Topic 1 - Atomic Structure & The Periodic Table in AQA GCSE Chemistry (Triple Higher). They cover specification points 4.1.3.1 & 4.1.3.2.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Group 1 metals are soft and have low strength, while transition metals are hard and strong.
In contrast to the single +1 ion formed by all Group 1 metals, transition metals can form _________ ____.
multiple ions
In the central block, between Group 2 and Group 3.
Transition metals are typically harder and stronger.
Mercury
Transition metals are much less reactive than Group 1 metals.
They react slowly or not at all.
Water
Iron(II) and Iron(III)
Copper(I) and Copper(II)
Unlike the typically white compounds formed by Group 1 metals, compounds containing transition metals are often what?
Coloured
Blue
They act as catalysts
A substance that increases the rate of a chemical reaction without being used up in the reaction itself.
Greater strength and greater hardness.
They lose electrons to form positive ions.
Solid
What property of transition elements is demonstrated by chromium forming Cr2+, Cr3+ and Cr6+ ions?
The ability to form ions with different charges.
This question is about metals and the reactivity series.
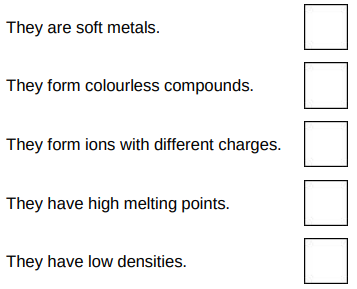
Which two statements are properties of most transition metals?
They form ions with different charges. They have high melting points.
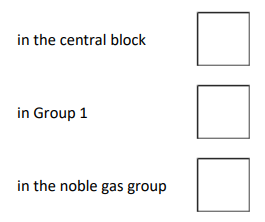
Where is copper in the periodic table?
In the central block
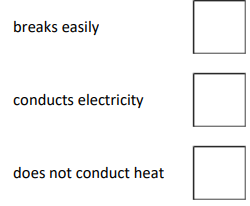
What is a property of copper?
Conducts electricity