aqa a level chemistry test tube reactions
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
differentiate between aqueous silver nitrate & other aqueous nitrates
- add hydrochloric acid
- silver nitrate forms white precipitate of AgCl
- other nitrates remain colourless
test for NH3(g)
- damp red litmus paper
- NH3 dissolves in H2O → paper turns blue
test for hydroxide (OH-)
- damp red litmus paper
- OH- are alkaline → paper turns blue
- further tests required to confirm if it is OH- as all alkalis turn red litmus paper blue
test for sulfates (SO4 2-)
- add HCl to remove carbonates
- add BaCl2
- sulfates form an insoluble white ppt of BaSO4
Ba2+(aq) + SO4 2-(aq) → BaSO4(s)
order of ion tests to prevent false positives
- carbonates using HCl
- sulfates using BaCl2
- halides using AgNO3 (& NH3)
samples of 1-chloropropane and ethanoyl chloride can be distinguished by the addition of an aqueous solution of silver nitrate. state what you would observe with each sample. (2)
- 1-chloropropane: no visible change
(accept 'small amount of precipitate' or 'precipitate forms slowly')
- ethanoyl chloride: white precipitate
(accept 'large amount of precipitate' or 'precipitate forms immediately')
test for halogenoalkane (HX)
- heat with NaOH(aq)
- acidify with HNO3
- add AgNO3
- precipitate of AgX formed
observations for the reaction: F-(aq) + AgNO3(aq)
forms soluble precipitate of AgF so solution is colourless
observations for the reaction: Cl-(aq) + AgNO3(aq)
- forms a white precipitate
Ag+(aq) + Cl-(aq) → AgCl(s)
observations for the reaction: Br-(aq) + AgNO3(aq)
- forms a cream precipitate
Ag+(aq) + Br-(aq) → AgBr(s)
observations for the reaction: I-(aq) + AgNO3(aq)
- forms a yellow precipitate
Ag+(aq) + I-(aq) → AgI(s)
observations for the reaction: Cl-(aq) + dilute NH3(aq)
- white ppt of AgCl dissolves & forms a colourless solution
AgCl(s) + 2NH3(aq) → Ag(NH3)2+(aq) + Cl-(aq)
observations for the reaction: Br-(aq) + concentrated NH3(aq)
- cream ppt AgBr dissolves & forms a colourless solution
AgBr(s) + 2NH3(aq) → Ag(NH3)2+(aq) + Br-(aq)
distinguish between AgCl & AgBr
- add dilute ammonia
- white ppt of AgCl dissolves
- cream ppt of AgBr remains as it doesn't dissolve
distinguish between AgBr & AgI
- add concentrated ammonia
- cream ppt of AgBr dissolves
- yellow ppt of AgI remains as it doesn't dissolve
observations for the reaction: Cl2(aq) + Br-(aq)
- solution turns from colourless to orange
Cl2 + 2Br- → 2Cl- + Br2
observations for the reaction: Cl2(aq) + I-(aq)
- solution turns from colourless to brown
Cl2 + 2I- → 2Cl- + I2
observations for the reactions: Br2(aq) + I-(aq)
- solution turns from colourless to brown
Br2 + 2I- → 2Br- + I2
reaction between sodium halides & conc sulfuric acid
NaX(s) + H2SO4(l) → NaHSO4(s) + HX(g)
distinguish between HCl(aq) & HNO3(aq)
- add silver nitrate
- HCl forms a white precipitate of AgCl
- HNO3 remains colourless (no visible change)
observations for the reaction between Cl- (NaCl) & conc H2SO4
- white misty fumes of HCl
NaCl + H2SO4 → NaHSO4 + HCl
observations for the reaction between Br- (HBr) & conc H2SO4
- orange vapour of Br2
2HBr + H2SO4 → Br2 + SO2 + 2H2O
observations for the reaction between I- (HI) & conc H2SO4
- yellow solid of I2
2HI + H2SO4 → I2 + SO2 + 2H2O
observations for the reaction between I- (HI) & SO2
- rotten egg smell from H2S
6HI + SO2 → H2S + 3I2 + 2H2O
flame colour for Ca2+
dark red
flame colour for Sr2+
red
flame colour for Ba2+
green
test for ammonium (NH4+)
- gently heat with NaOH(aq)
- ammonia (NH3) gas produced
- test for NH3 gas with damp red litmus paper (turns blue)
explain why it is not possible to distinguish between separate solutions of sodium nitrate and sodium fluoride by the addition of silver nitrate solution. (1)
AgF is soluble (so solution remains colourless)
draw the structure of ethanoyl choride. predict what, if anything, you would observe when ethanoyl chloride is added to aqueous silver nitrate. (2)
vigorous or violent or exothermic reaction or fumes or white precipitate formed immediately
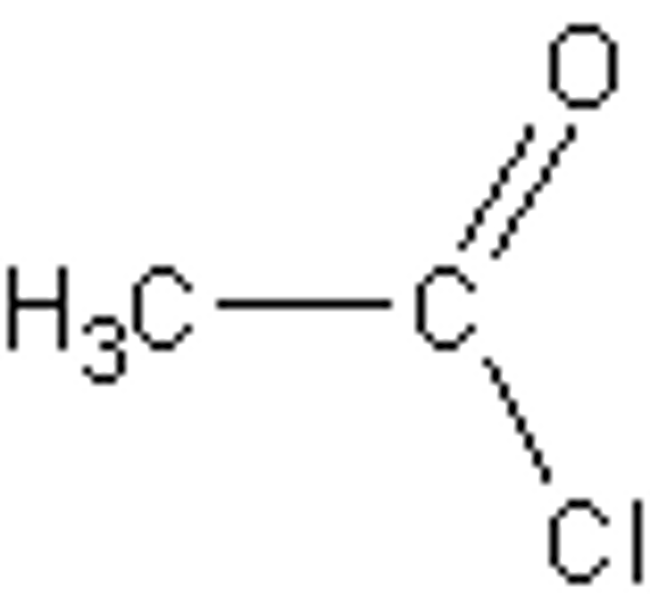
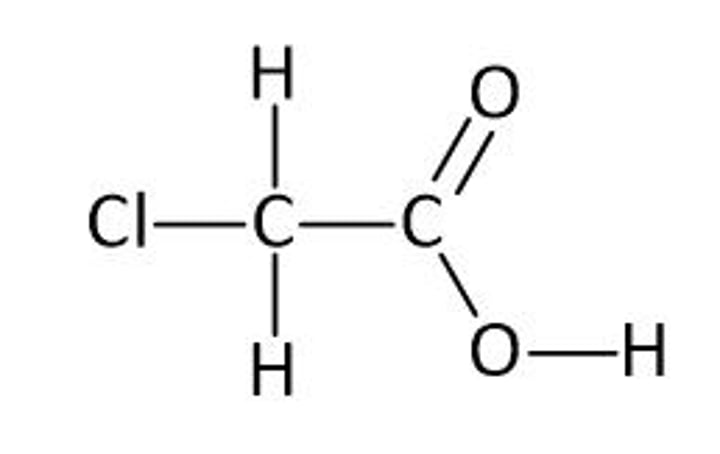
draw the structure of chloroethanoic acid. predict what, if anything, you would observe when chloroethanoic acid is added to aqueous silver nitrate. (2)
- no immediate precipitate or reaction
OR
- white precipitate formed very slowly
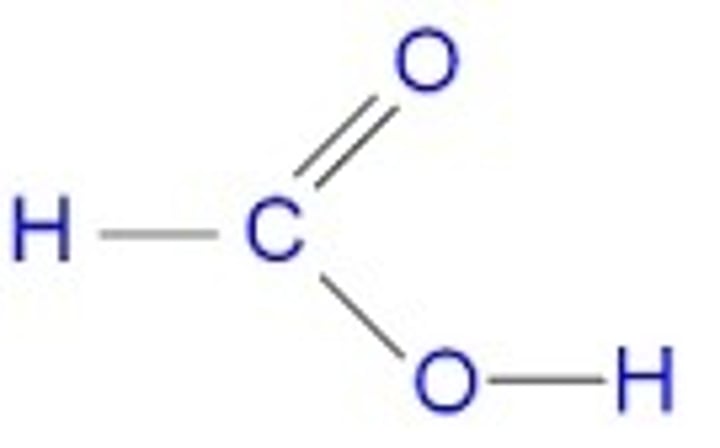
draw the structure of methanoic acid. by reference to this structure, suggest why a silver mirror is formed when this acid reacts with Tollens' reagent. (2)
methanoic acid contains an aldehyde group
test for alkenes or C=C
- add bromine water
- turns colourless
distinguish between alkene & alkane
- add bromine water
- alkene turns from orange to colourless
- alkane stays orange (no visible change)
test for primary & secondary alcohols
- add acidified potassium dichromate
- colour change from orange to green
distinguish between primary/secondary alcohol & tertiary alcohol
- add acidified potassium dichromate
- primary/secondary alcohol turns from orange to green
- tertiary alcohols are not oxidised so stay orange (no visible change)
alternative test for alcohols
- add acidified potassium manganate(VII)
- primary/secondary alcohols turn from purple to colourless
- tertiary alcohols remain purple (no visible change)
test for aldehydes with tollen's reagent
- aldehydes form a silver mirror
- ketones remain colourless (no visible change)
test for aldehydes with fehling's solution
- aldehydes form a brick red precipitate
- ketones remain blue (no visible change)
test for carboxylic acid
- add sodium carbonate
- effervescence as carbon dioxide gas formed
test for carbon dioxide (after testing for carboxylic acid)
- bubble through limewater
- limewater turns cloudy