Physical Changes and Chemical Changes 2022
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
physical change
A type of change that involves the physical properties of a substance. No new matter is made.

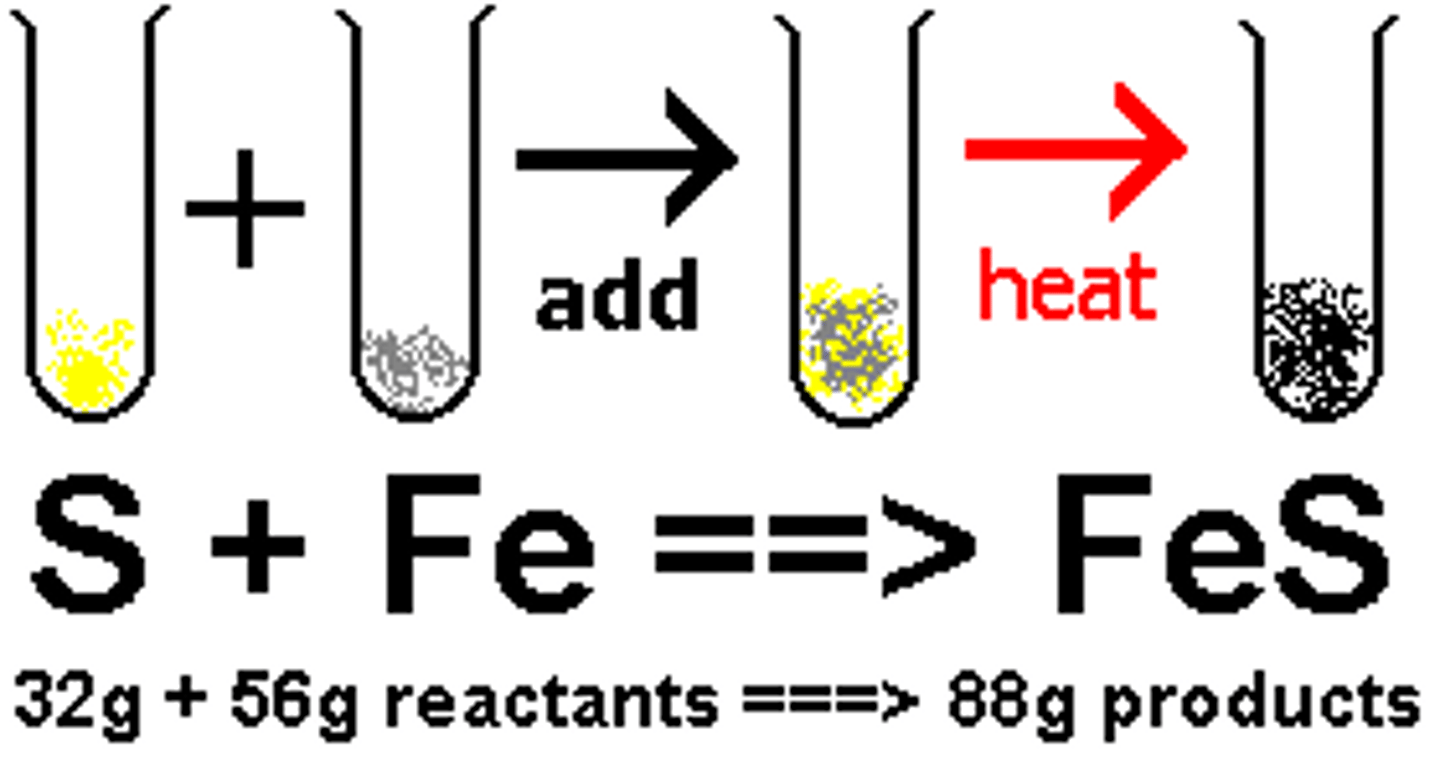
chemical change
Process by which substances are changed into different substances with different properties

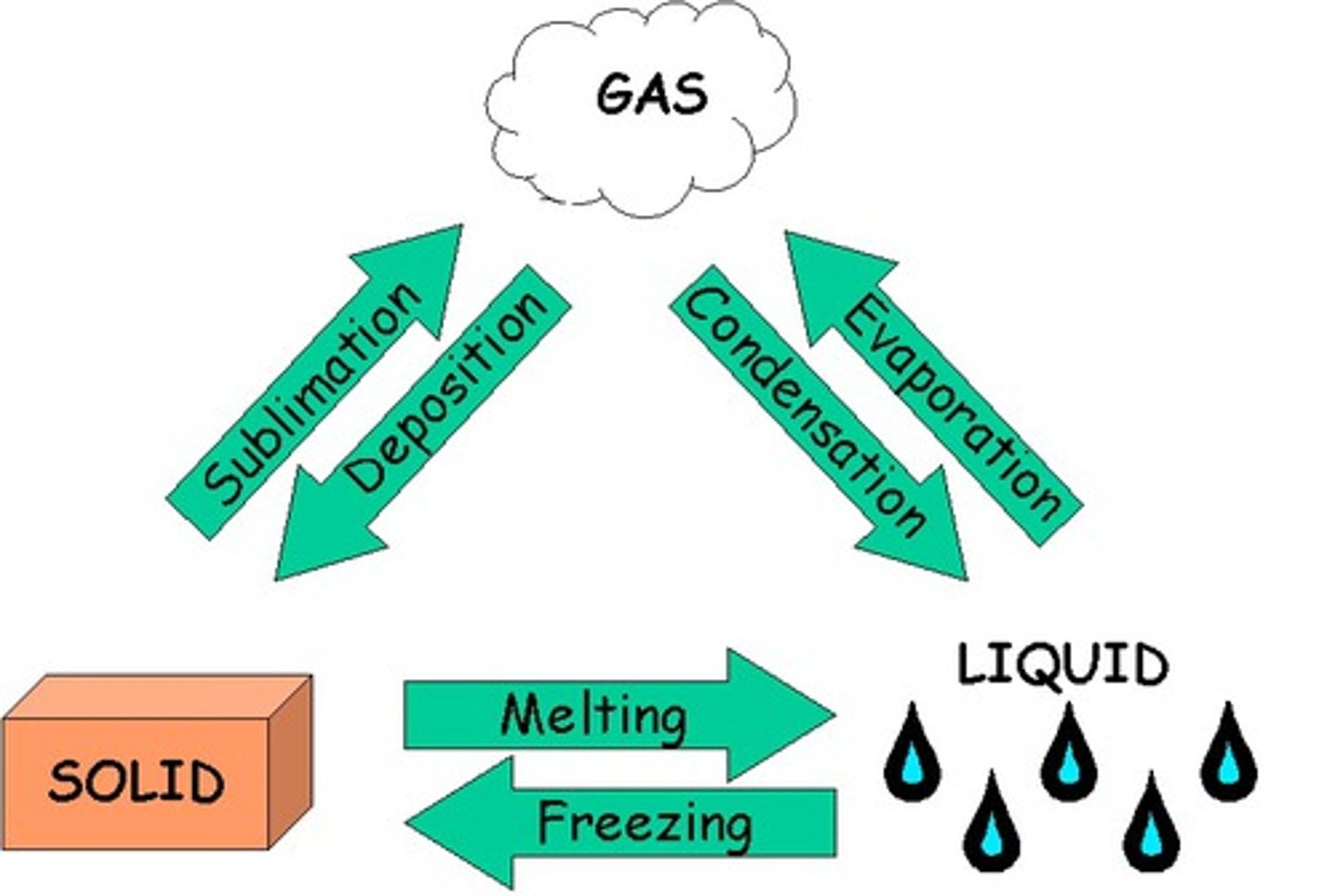
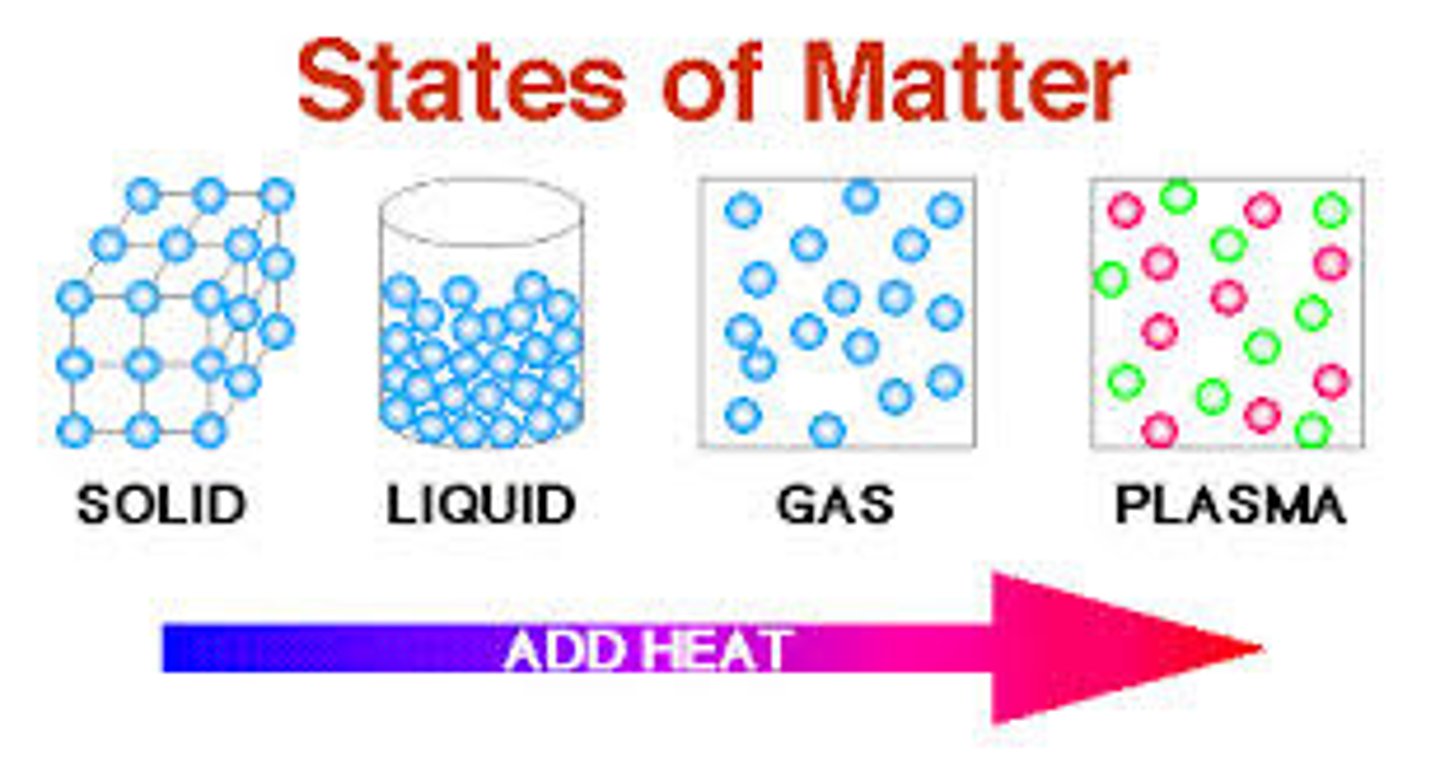
phase change
A physical change that changes the state of matter (solid, liquid, gas, plasma).
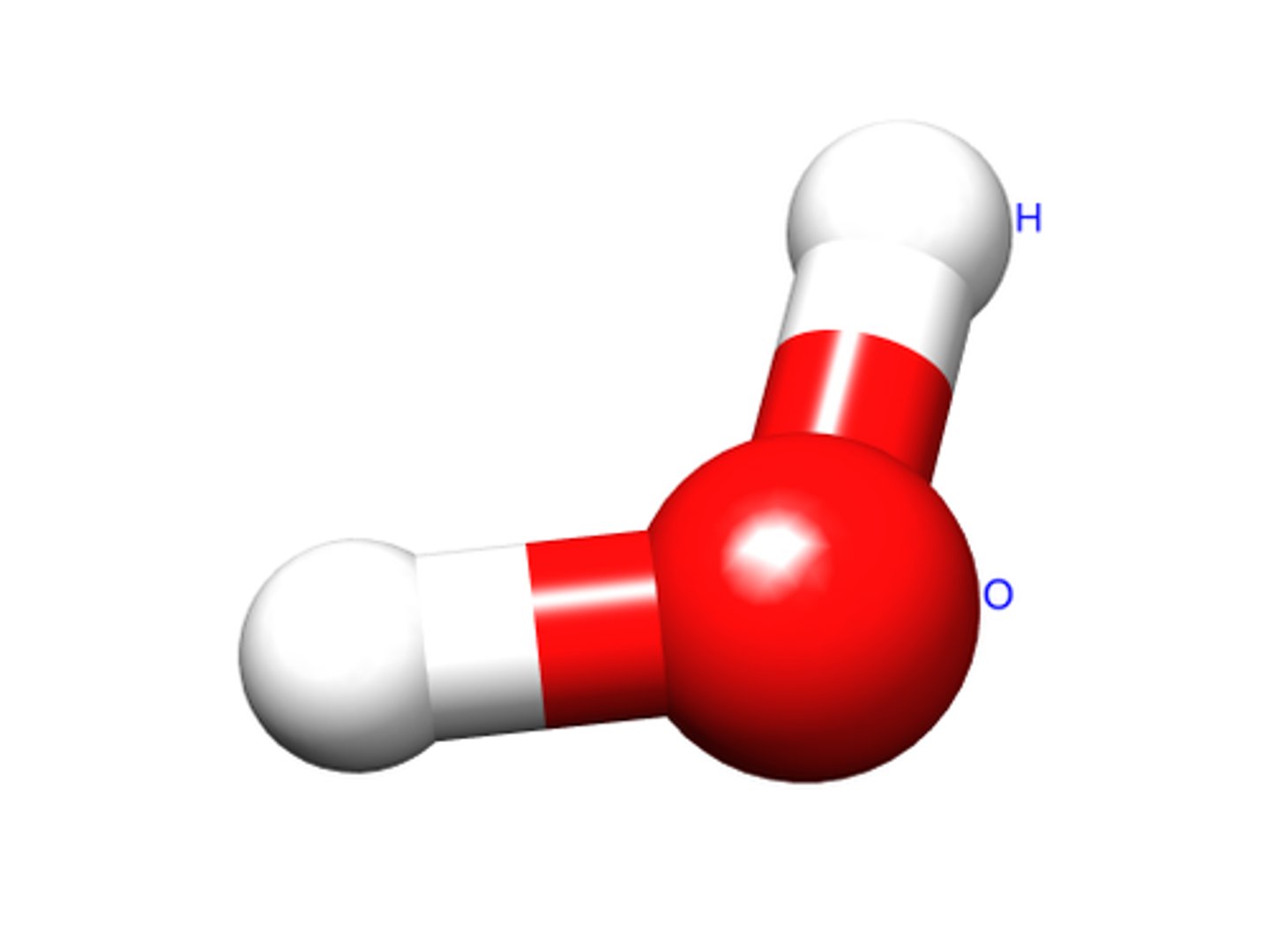
molecule
made up of two or more atoms combined chemically
Compound
A substance made up of atoms of two or more different elements joined by chemical bonds / a type of molecule
mass
a measure of the amount of matter present

Atom
the basic unit of a chemical element / contains protons, neutrons and electrons
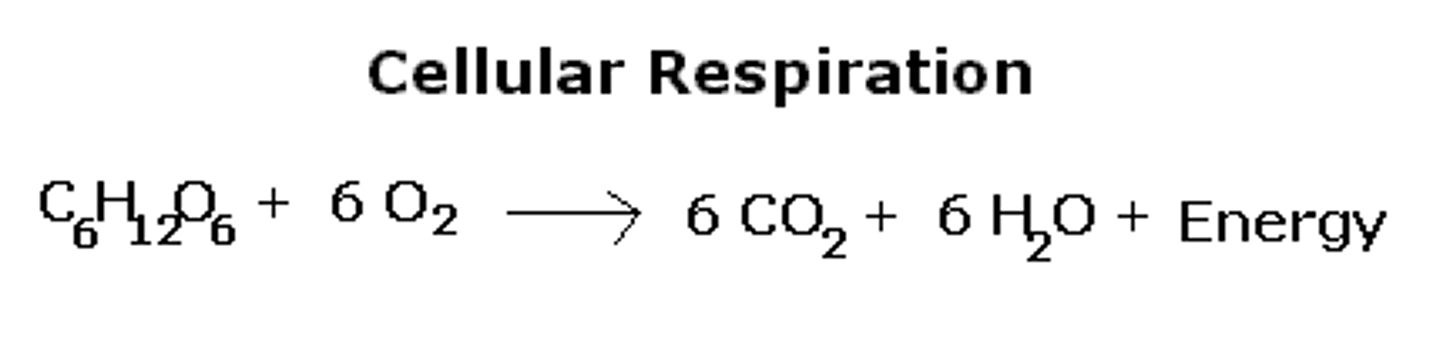
Mixture
A combination of two or more substances that are not chemically combined
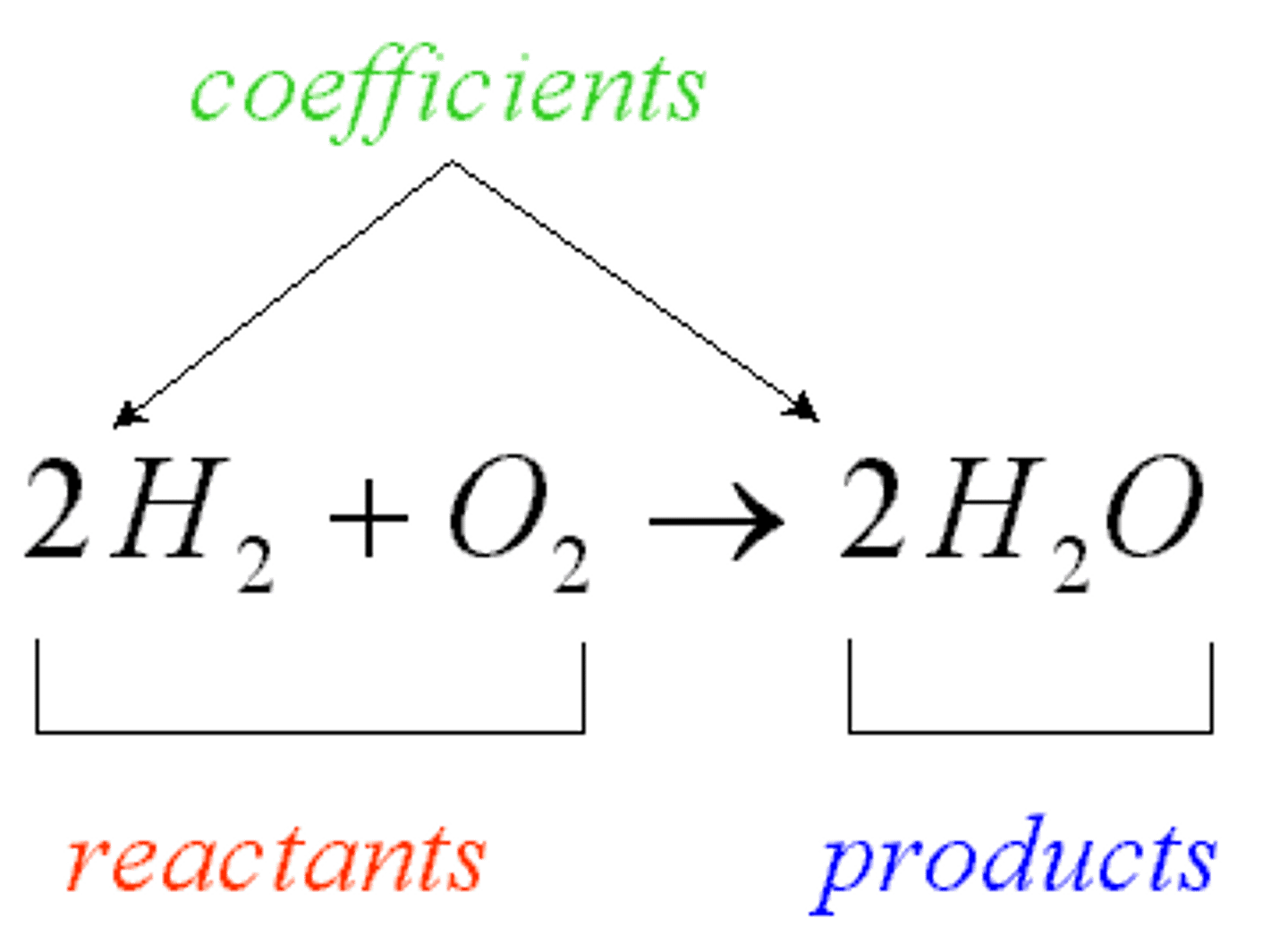
matter
anything that has mass and takes up space
chemical property
a property of matter that describes a substance's ability to participate in chemical reactions
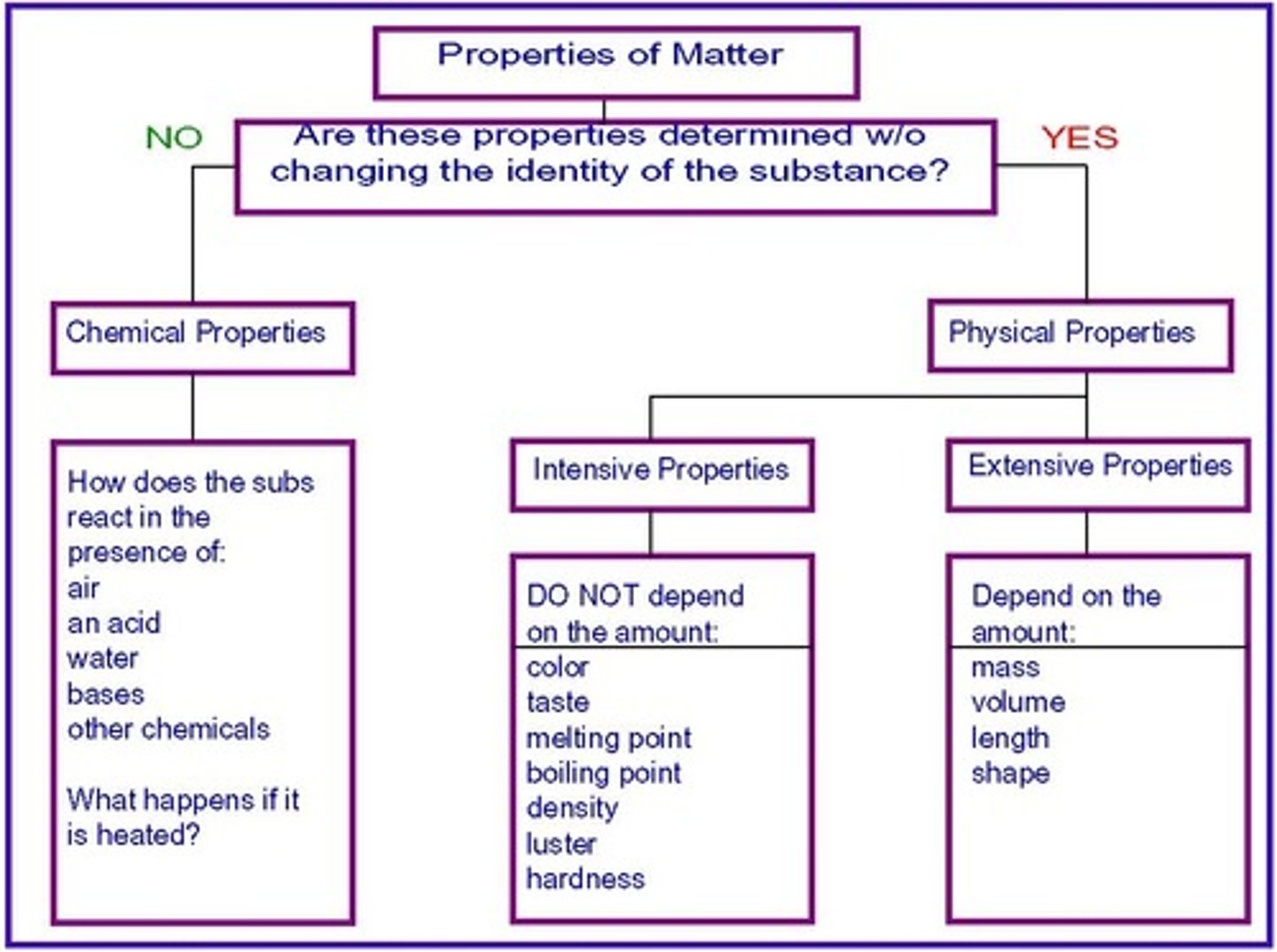
physical property
A characteristic of a pure substance that can be observed without changing it into another substance

Reactivity
the property that describes how readily a substance combines chemically with other substances to make new matter
pure substance
A substance made of only one kind of matter and having definite properties.
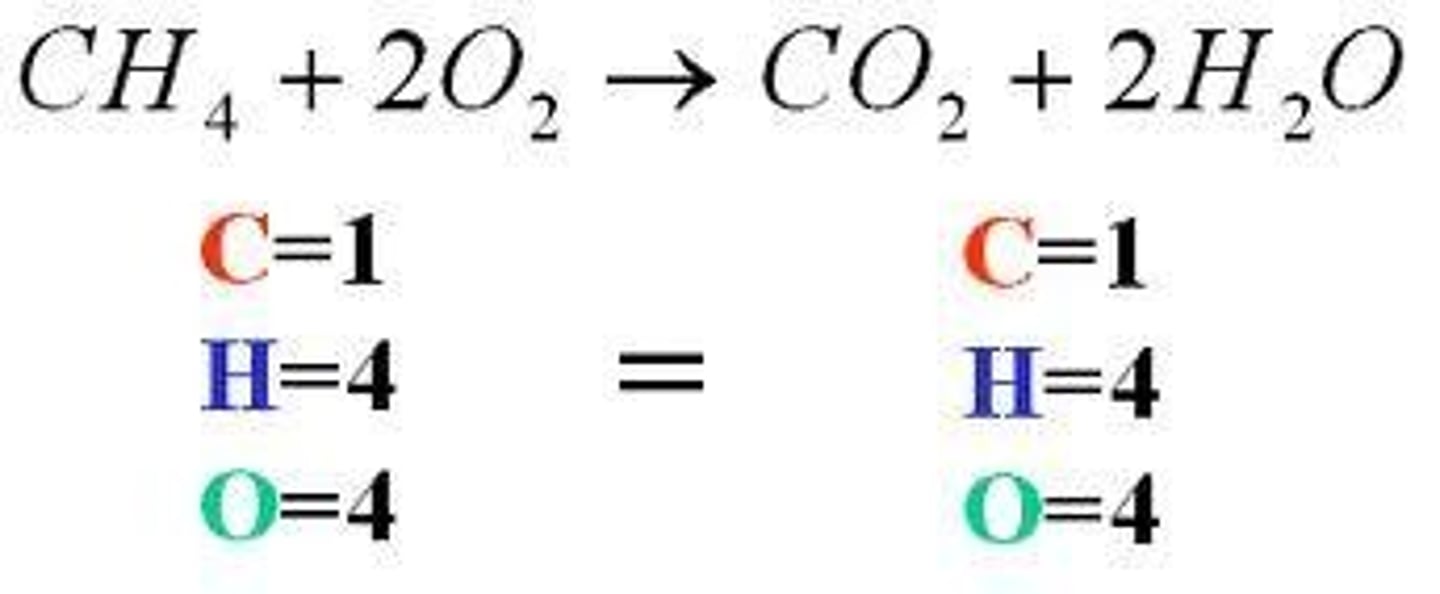
Indicators of a chemical change/reaction.
Gasses formed, heat changes, light, or sound production. This occurs as a result of new matter being made (atoms are rearranged and compounds broken)
change in shape, size, weight, density OR phase
types of physical changes
Solubility
the ability of one substance to dissolve in another at a given temperature and pressure
particle
often referencing components of atoms, or other small types of matter that have unique physical an chemical properties / from subatomic particles, such as electrons, to particles large enough to be seen, such as particles of dust floating in sunlight
crystal
a solid in which the atoms are arranged in a pattern that repeats again and again
Flammability
the ability of a substance to burn
units for density
g/mL or g/cm3
In a density column, these substances will be near the top
ones that are less dense
In a density column, these substances will be near the bottom
ones that are more dense
Density
mass/volume
melting point
the temperature at which a given solid will melt. This is a physical property.