H2O Unit - Chemistry
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
Define specific heat capacity
The amount of energy required to raise the temperature (usually 1 degrees C) of a set amount of substance (usually 1 g).
2
New cards
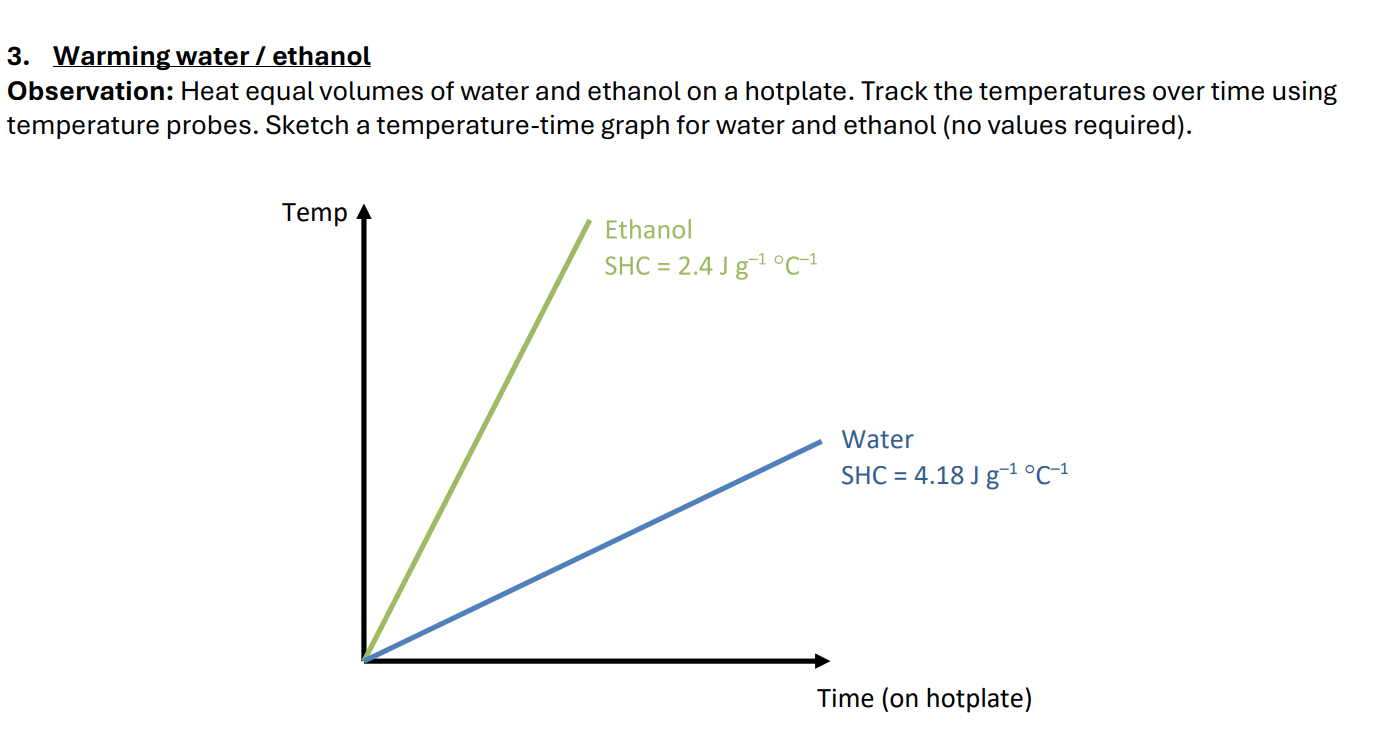
Does water have a high or low specific heat capacity? How does the demonstration show this?
High. It required more heat energy (had to absorb heat energy for longer) to bring the same volume to the same temperature.
3
New cards
Explain why water has a relatively high specific heat capacity.
A relatively large amount of energy is absorbed to break or loosen hydrogen bonds between water molecules.
4
New cards
ayay
ayya