covalent bonds
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
What is covalent bonding
The sharing of electrons
A covalent bond is formed when a pair of electrons is shared between atoms . Atoms share electrons with each other to get a full outer shell and be stable
How are covalent bonding represented
Through dot and cross diagrams , displayed formulas and. 3D models
Where do covalent bonds Accor
Between non metal atoms
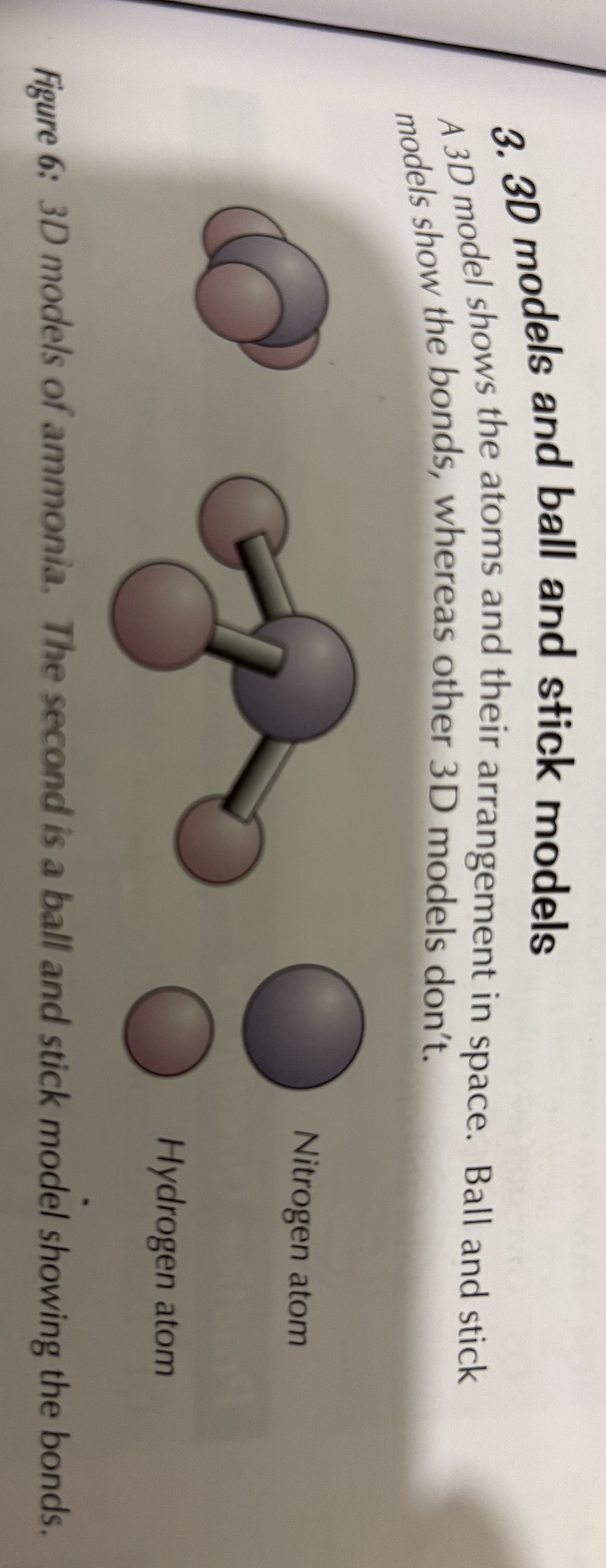

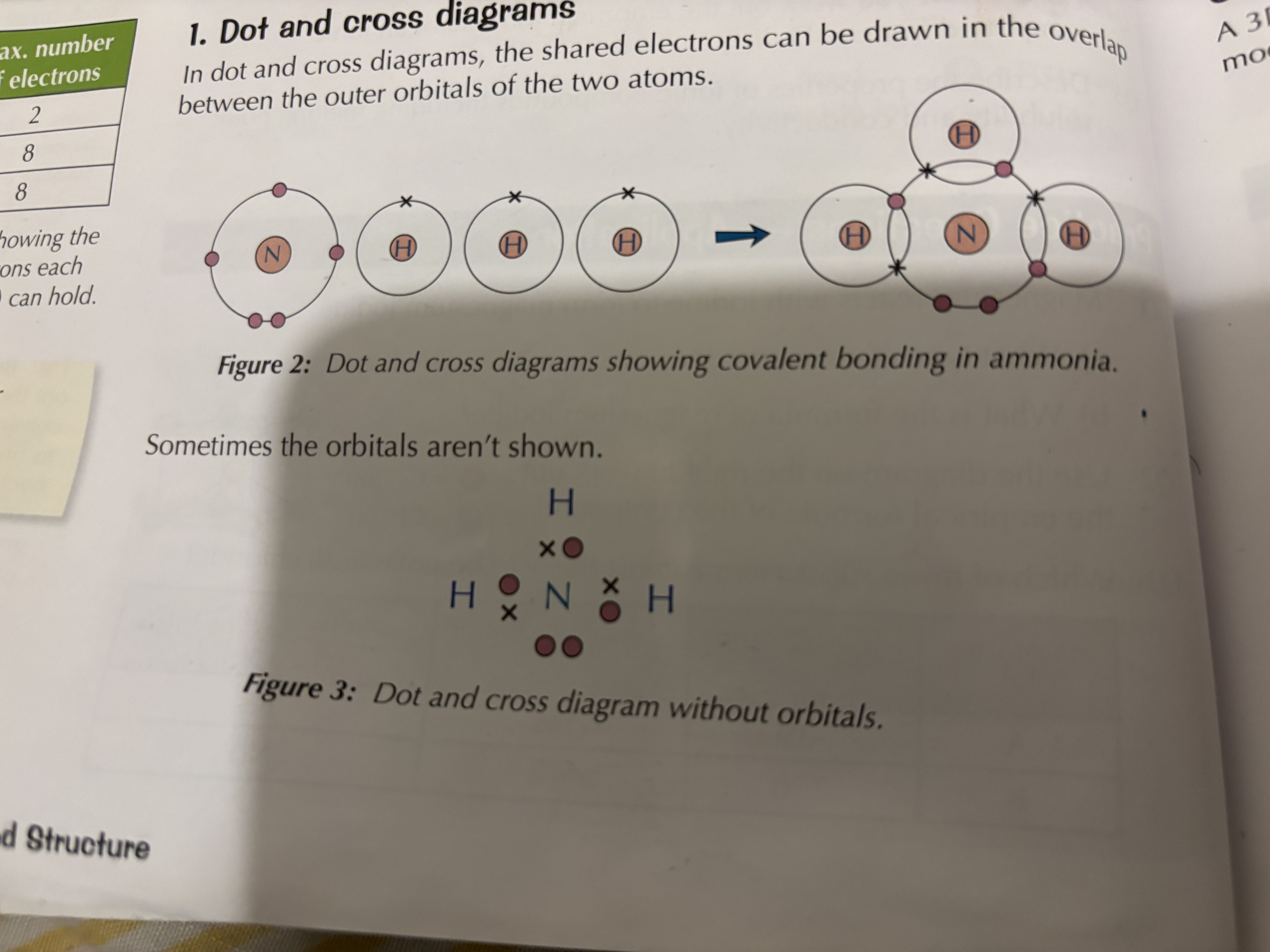

What are the disadvantages of using dot and cross diagrams, displayed diagrams and 3 D models to show covalent bonds
Dot and cross-they don’t show the relative sizes of the atoms or how the atoms are arranged in space
Displayed formulas-they don’t show the 3-D structure of the molecule or which atoms the electrons in the covalent bond have come from
3D models -3-D models don’t show bonds and they can quickly get confusing for large molecules where there are a lot of atoms to include. They don’t show where the electrons in the bonds have come from as well.
Finding molecular formula
