MOR Chemistry Module 2
1/94
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
95 Terms
isotopes
atoms of the same element with different neutron numbers + different mass numbers
relative atomic mass
Ar
weighted mean mass of an atom of an element, relative to 1/12th mass of a carbon-12 atom
why may the Ar of a sample of an element be different from the Ar on the periodic table?
sample may not accurately reflect the relative abundances of all isotopes - the periodic table may use a different sample
example: higher abundance of 1 isotope will influence average mass/Ar
relative isotopic mass
mass of an isotope of an element, relative to 1/12th mass of a carbon-12 atom
relative molecular mass
weighted mean mass of a molecule, relative to 1/12th mass of a carbon-12 atom
relative formula mass
calculation
sum of all RAMs of elements, as given in a formula of non-molecular compounds
calculation: sum up all RAMs of elements
relative mass of an electron
1/1835
relative atomic mass calculation
see image
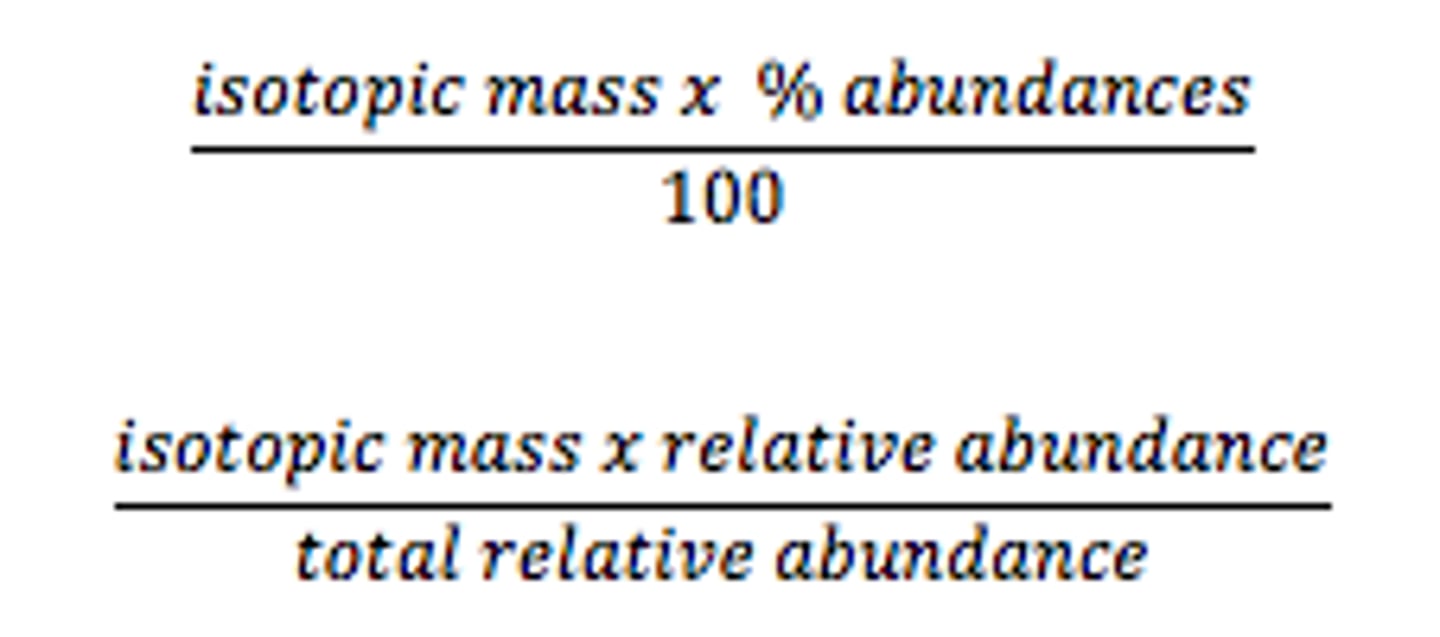
acid
proton donor (H+)
alkali
A base that is soluble in water
releases OH- ions in solution
base
can neutralise an acid to produce a salt
proton acceptor
disproportionation reaction
same element is simultaneously oxidised and reduced in the same reaction
aufbau principle
lowest energy levels are occupied first
pauli exclusion rule
each orbital cannot contain more than 2 electrons, with opposing spins
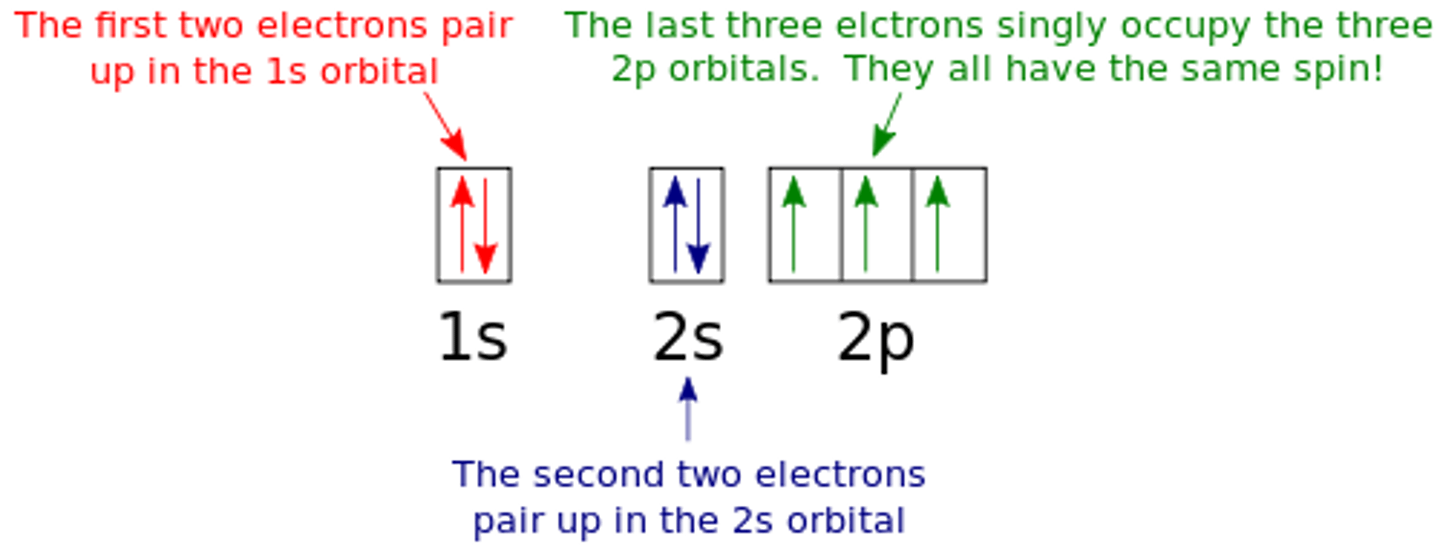
hund's rule
single electrons occupy all empty orbitals first, before forming pairs
because electrons repel
4 common acids
nitric acid HNO3
ethanoic acid CH3COOH
sulfuric acid H2SO4
hydrochloric acid HCl
4 common bases
ammonia NH3
potassium hydroxide KOH
sodium hydroxide NaOH
+ all carbonates (e.g. Na2CO3)
strong acid
more H+ ions fully dissociate and completely ionise
more ions left in solution
less molecules left in solution
lower pH
weak acid
less H+ ions fully dissociate and only partly ionise
less ions left in solution
more molecules left in solution
higher pH
neutralisation reaction
H+ ion in the acid is replaced by metal ion or NH4 + ion
acid + base --> salt + water
example: HCl + NaOH --> NaCl H2O
metal carbonate + acid
salt + water + carbon dioxide
metal + acid
salt + hydrogen
acid + base
salt + water
neutralisation ionic equation
H+ (aq) + OH- (aq) >>> H2O (l)
how is a salt formed?
when H+ ion is replaced by a metal ion or NH4 + ion
oxidation
loss of electrons
oxidation number increases
reduction
gain of electrons
oxidation number decreases
describe the currently accepted model of the atom
electrons share fixed energies
electrons move around nucleus in energy levels/shells
what is principal quantum number
each energy shell is assigned a principal quantum number (n)
how does principal quantum number change?
further away from nucleus = larger principal quantum number
s-subshell
1 orbital, 2 electrons
p-subshell
3 orbitals, 6 electrons
d-subshell
5 orbitals, 10 electrons
f-subshell
7 orbitals, 14 electrons
shell 1
1s = 2 electrons
shell 2
2s, 2p = 8 electrons
shell 3
3s, 3p, 3d = 18 electrons
shell 4
4s, 4p, 4d, 4f = 32 electrons
s-orbital shape
spherical
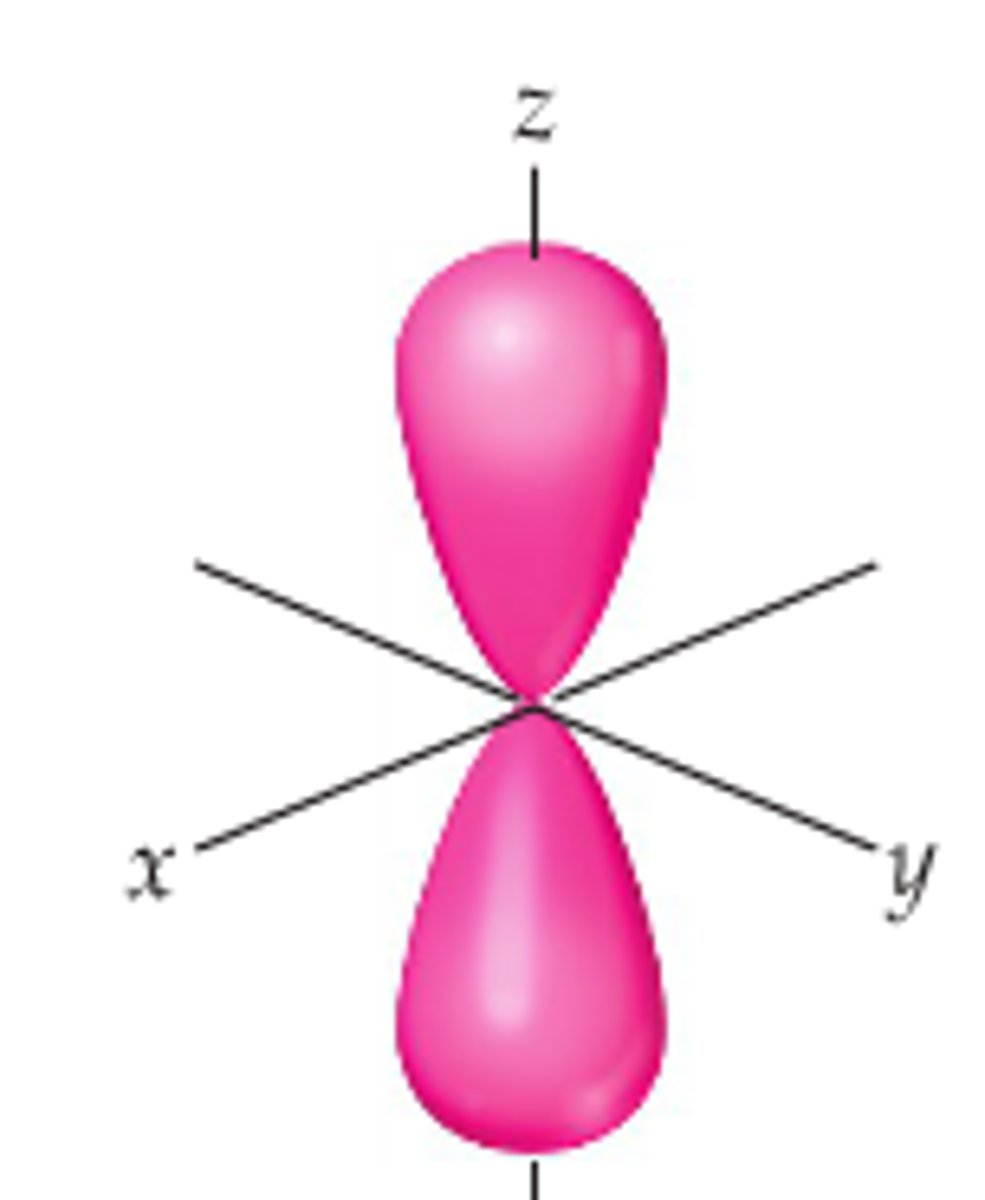
p-orbital shape
dumbell shape
maximum number of electrons in an orbital
2 electrons
what is an orbital?
space an electron moves in
pair of 2e- in an orbital have opposing spins
what are opposing spins
pair of electrons spin in opposite directions (spin-pairing)
2 electrons can only occupy the same orbital if they have opposing spins
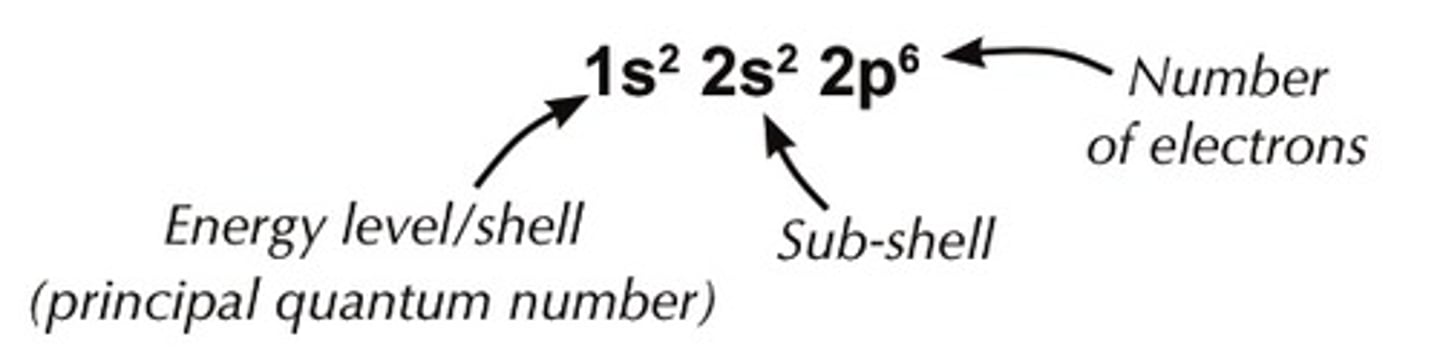
subshell notation
example in image
box and arrow model
each box = 1 orbital
each arrow = 1 electron
up/down directions of arrows represent opposing spins of electrons
electron energy level diagrams
shows energies of electrons in different orbitals
expresses:
- number of electrons
- electron arrangement
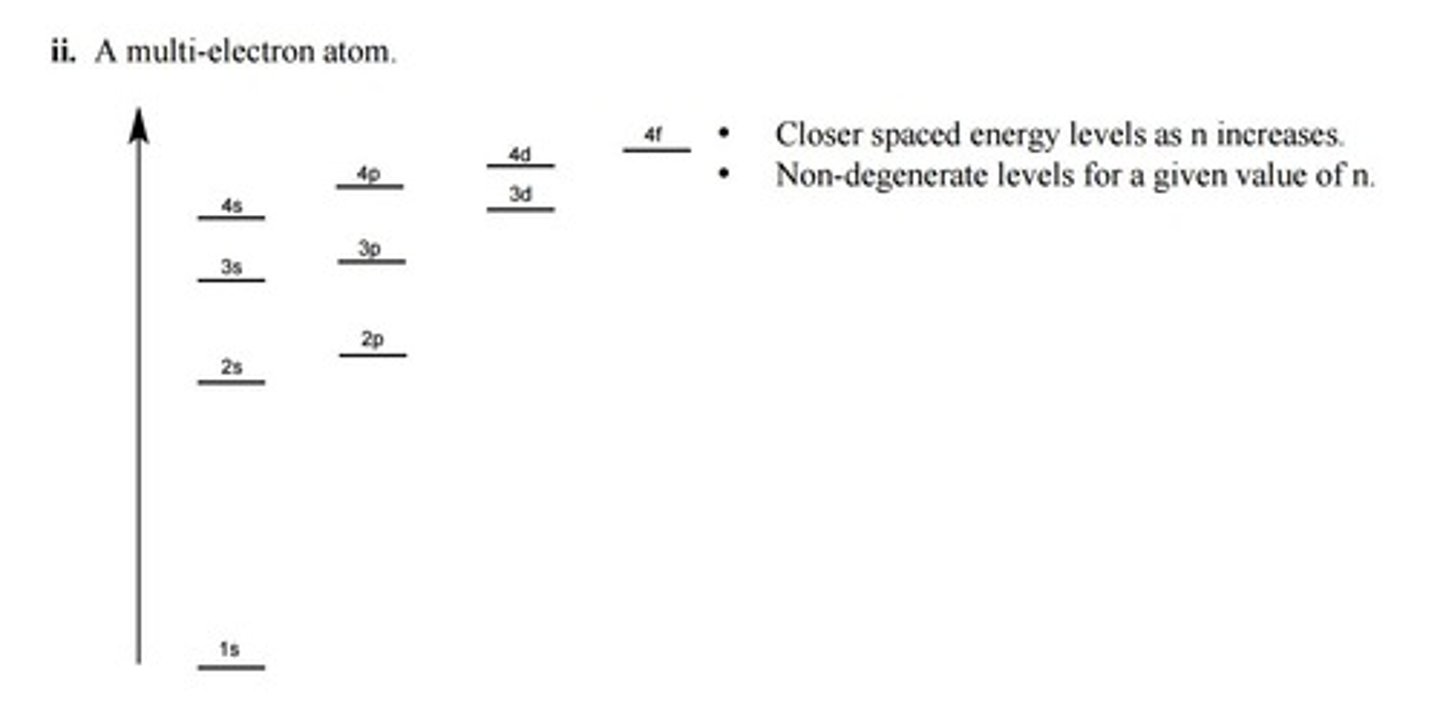
order of filling
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p

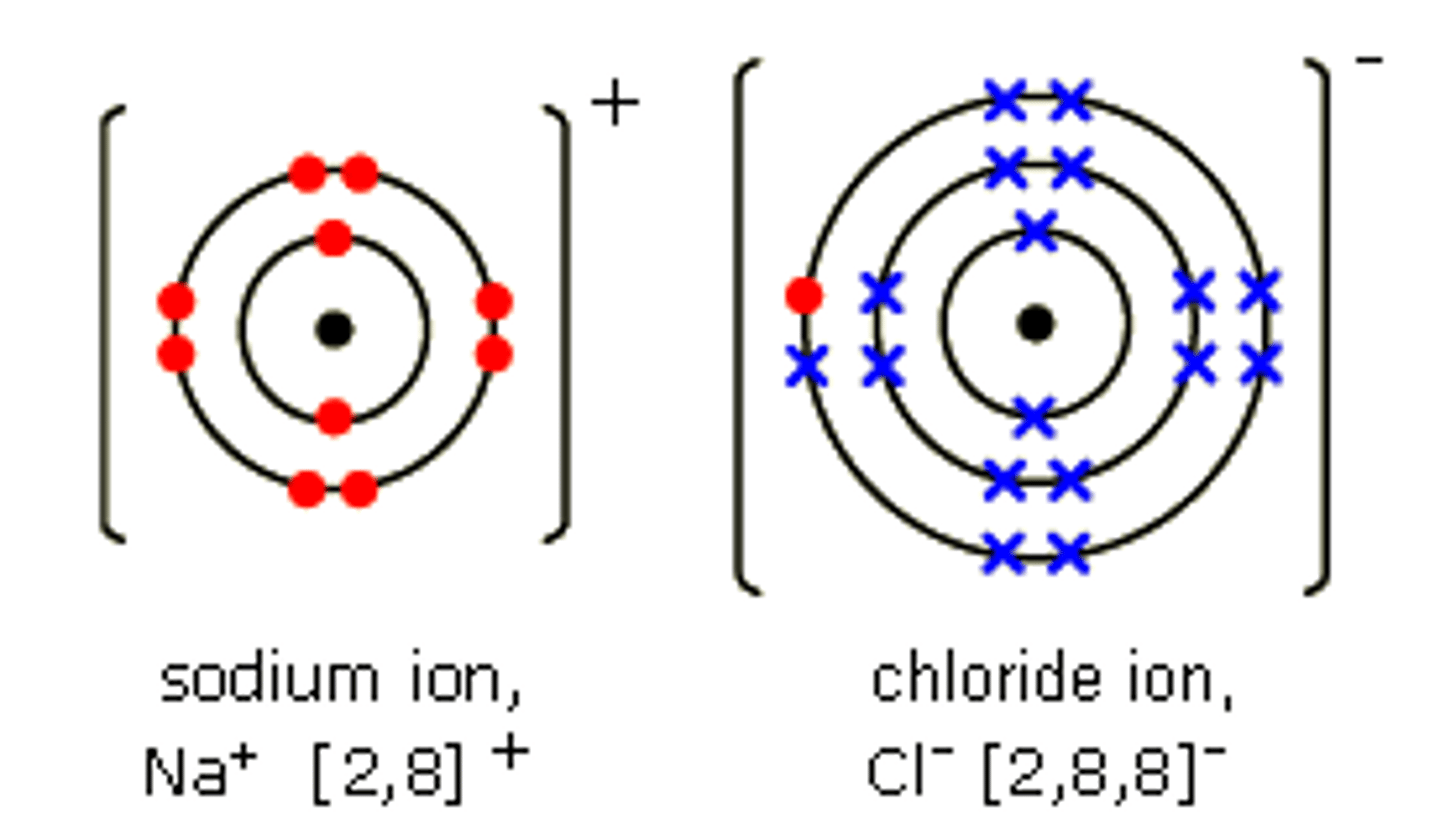
ionic bond
the electrostatic attraction between oppositely charged ions
dot and cross diagram example
image
giant ionic lattice
regular structure
'giant' because the same basic units are repeated over and over
why do giant ionic lattices form?
every ion is electrostatically attracted to oppositely charged ions in all directions
electrical conductivity of ionic compounds
conduct electricity when molten/in solution
cannot conduct as solids, because ions are in fixed positions (due to strong electrostatic forces), so cannot carry a charge
melting/boiling points of ionic compounds
high melting/boiling points, because giant ionic lattices are held together by strong electrostatic forces
a lot of energy is required to overcome these forces
solubility of ionic compounds
usually dissolve in water
water is polar (partly charged)
water molecules PULL ions away from the lattice = dissolves
define a compound
2+ atoms bonded together
covalent bond
the strong electrostatic attraction between a shared pair of electrons and the nuclei of the bonded atoms
single covalent bond
only 1 pair of electrons is shared
1e- is donated to bonding pair
double or triple covalent bonds
2 or 3 pairs of electrons are shared between atoms
describe a 'stable arrangement'
outer shell USUALLY filled to 8 electrons (octet)
+ exceptions exist
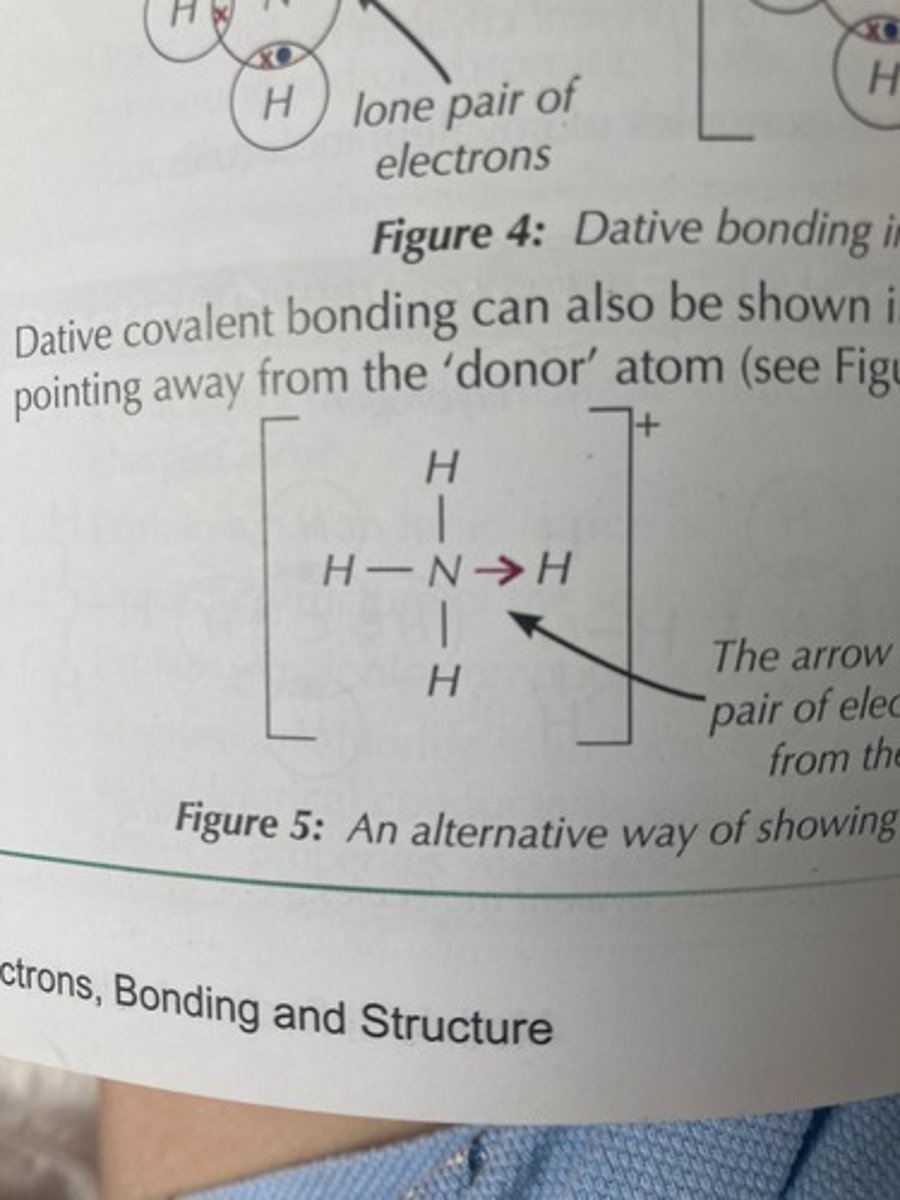
dative (coordinate) covalent bond
1 atom provides both electrons in the shared pair
expressing dative covalent bonds in diagrams
image
special exceptions to octet in outer shell
some compounds use d-orbitals to 'expand their octet' (can contain more than 8 electrons in outer shell) (e.g. SF6)
some compounds have less than 8 electrons in their outer shell (e.g. BF3)
radical
contains a single unpaired electron
very reactive
determining covalent bond strength
average bond enthalpy
stronger bond = more energy needed to break bond = higher bond enthalpy value
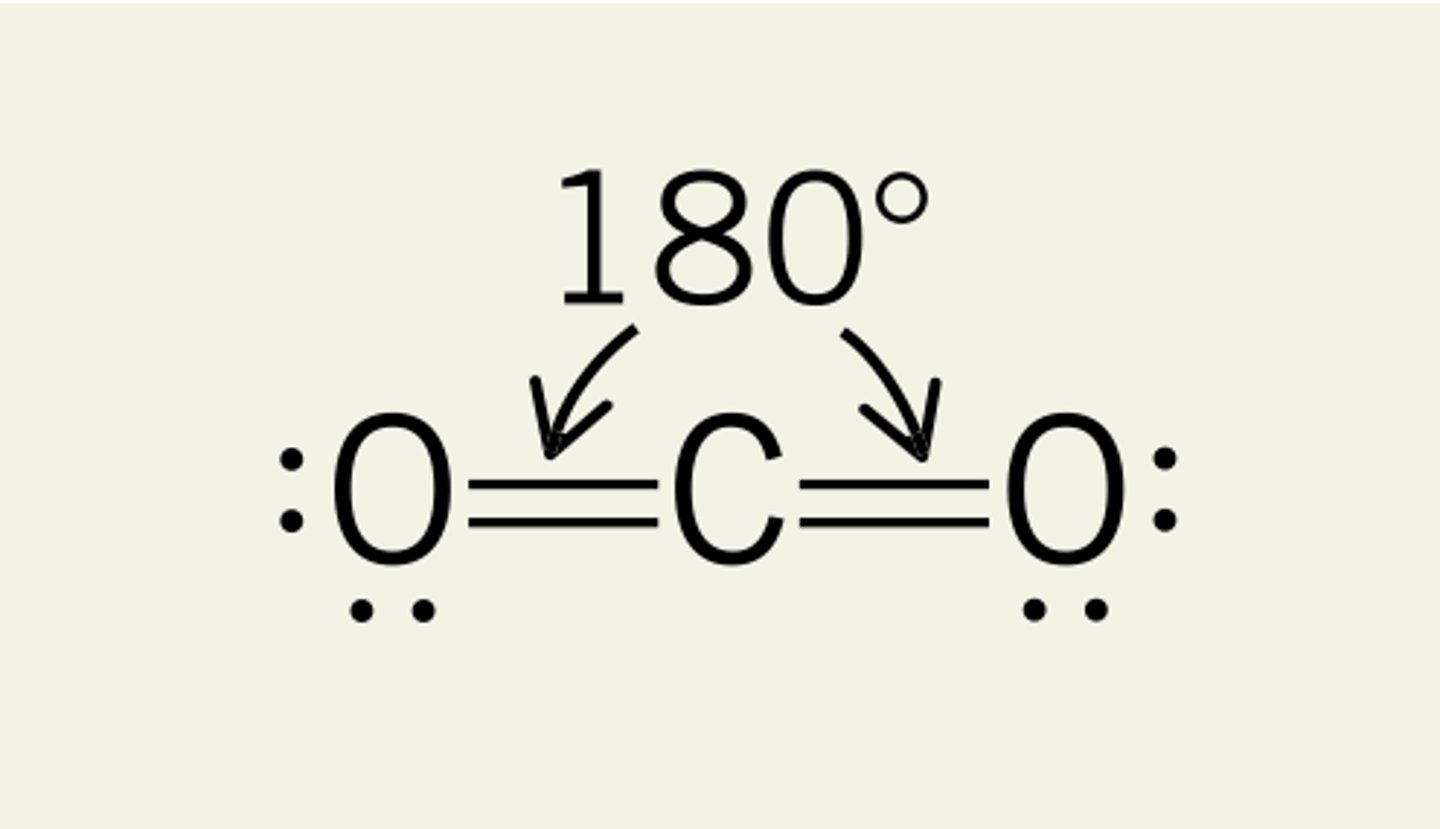
linear
2 bonding regions
no lone pairs
180 degrees
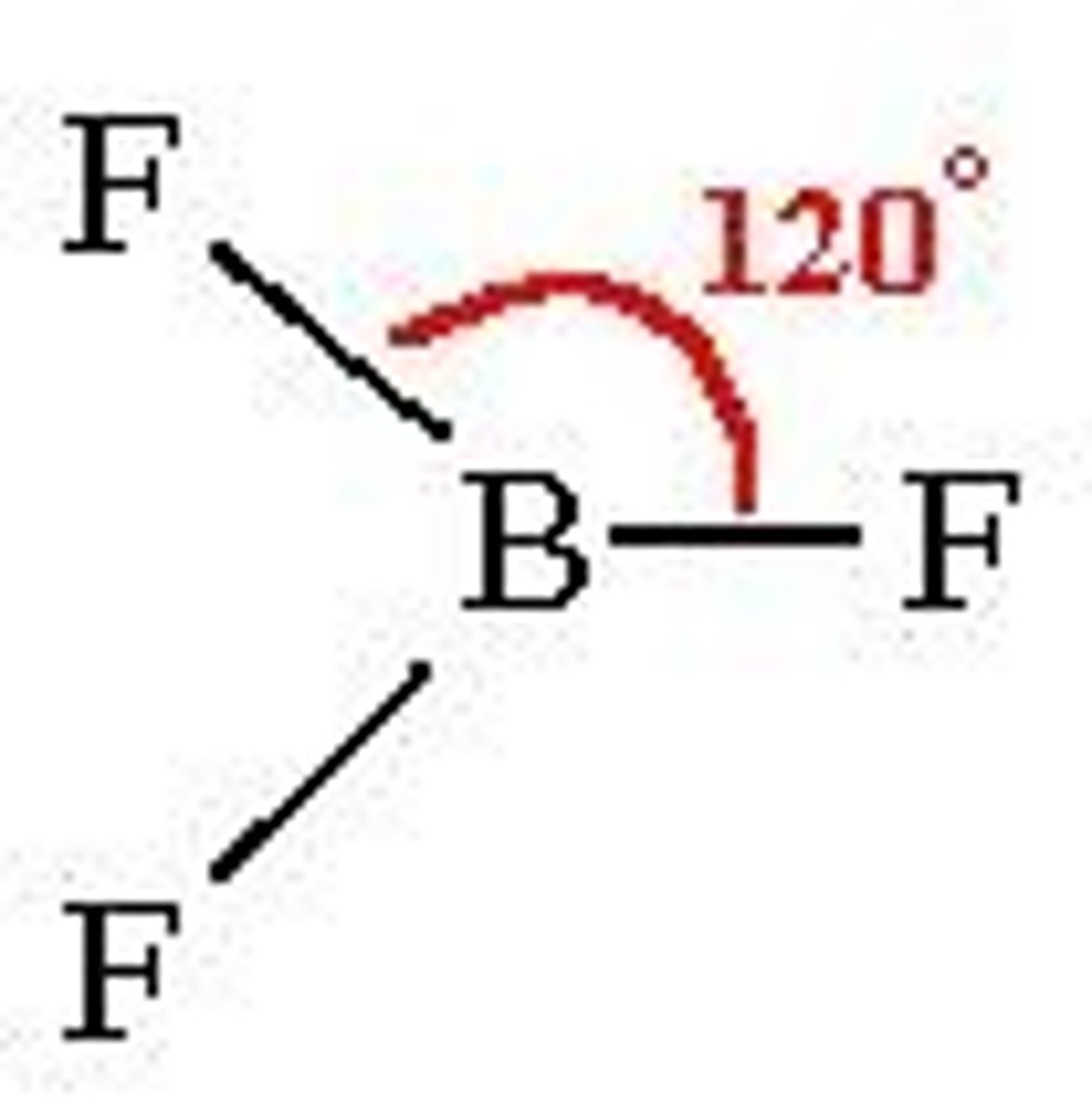
trigonal planar
3 bonding regions
no lone pairs
120 degrees
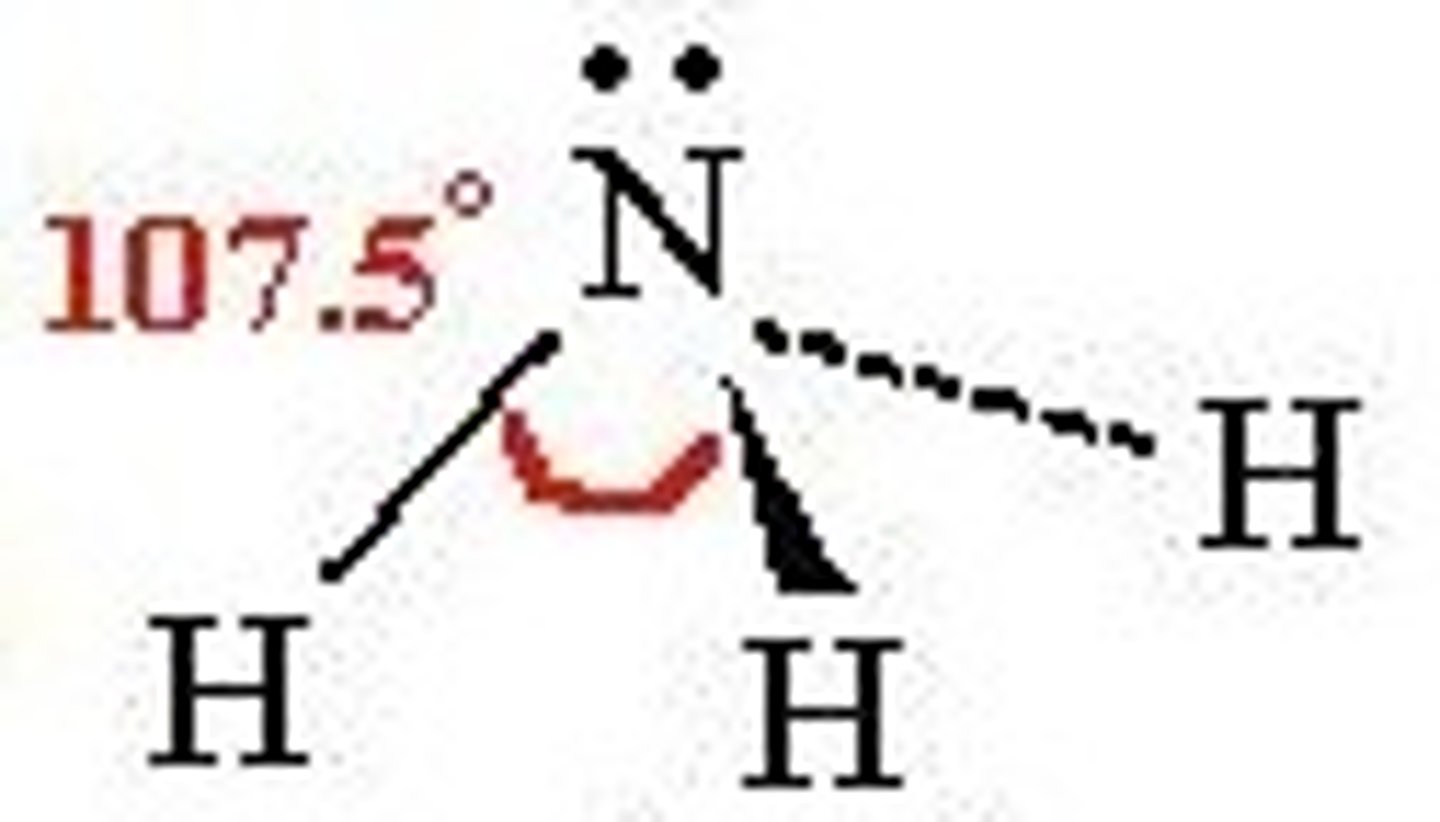
trigonal pyramidal
3 bonding regions
1 lone pair
107 degrees
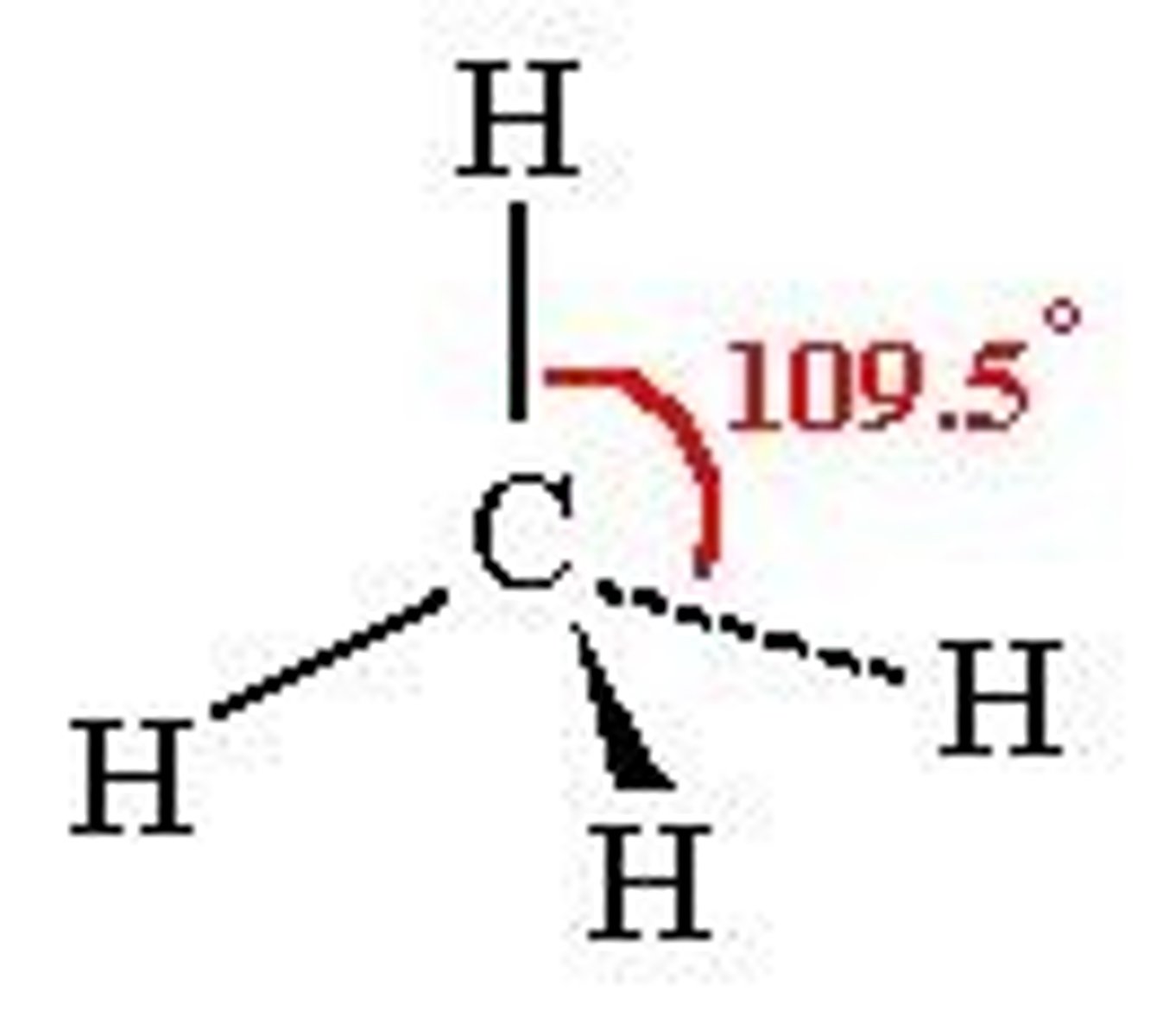
tetrahedral
4 bonding regions
no lone pairs
109.5 degrees
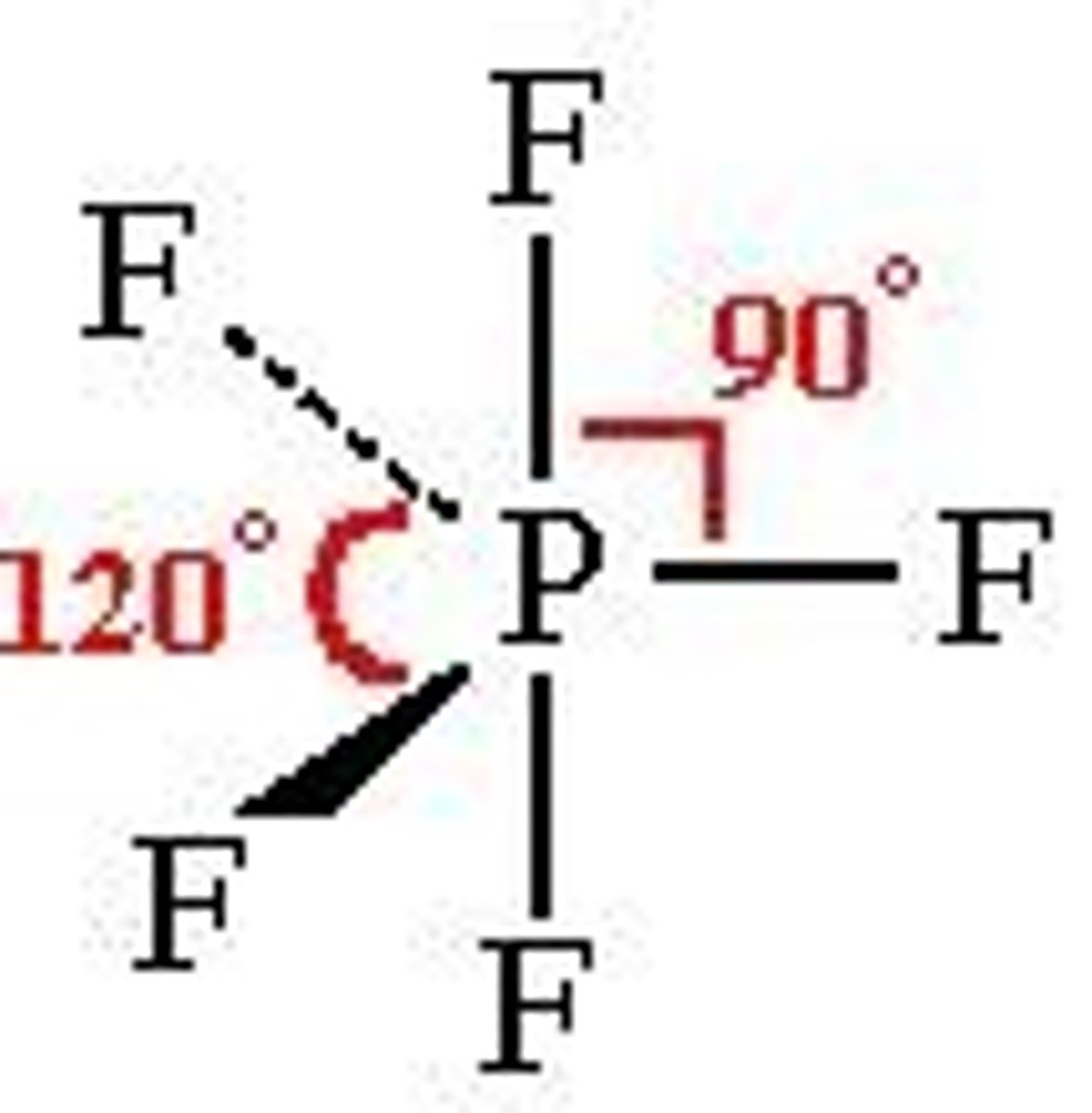
trigonal bipyramidal
5 bonding regions
no lone pairs
120 + 90 degrees
non-linear
2 bonding regions
2 lone pairs
104.5 degrees
octahedral
6 bonding regions
no lone pairs
90 degrees
repulsion of lone pairs
repel more
repulsion of bonded regions
repel equally
drawing shapes of molecules
image
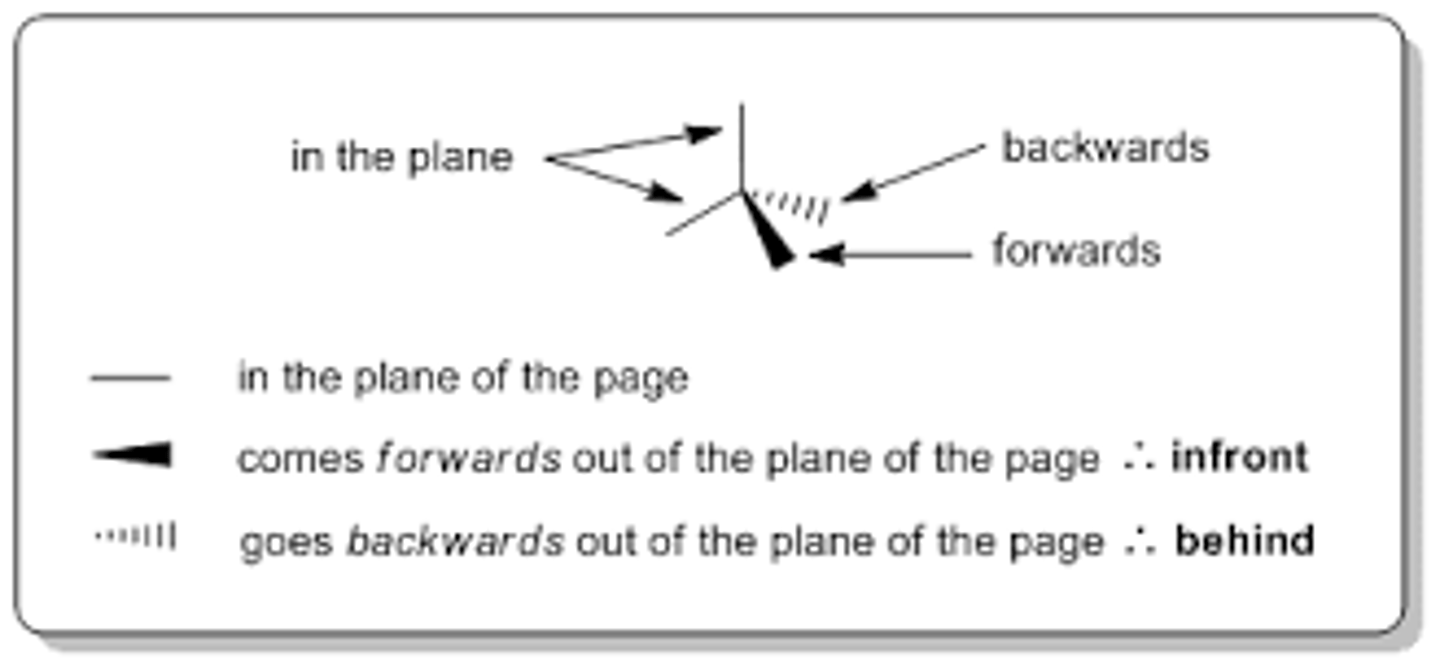
repulsion in shapes of molecules
pairs of electrons repel
repel to become as far from each other
assumed shape minimises repulsion
electronegativity
the ability of an atom to attract electrons in a covalent bond
what does a dipole contain?
both δ+ and δ- ends
if both ends were the same delta charge, then they would cancel out = no dipole
trend in electronegativity
increases as we move towards F on Periodic Table
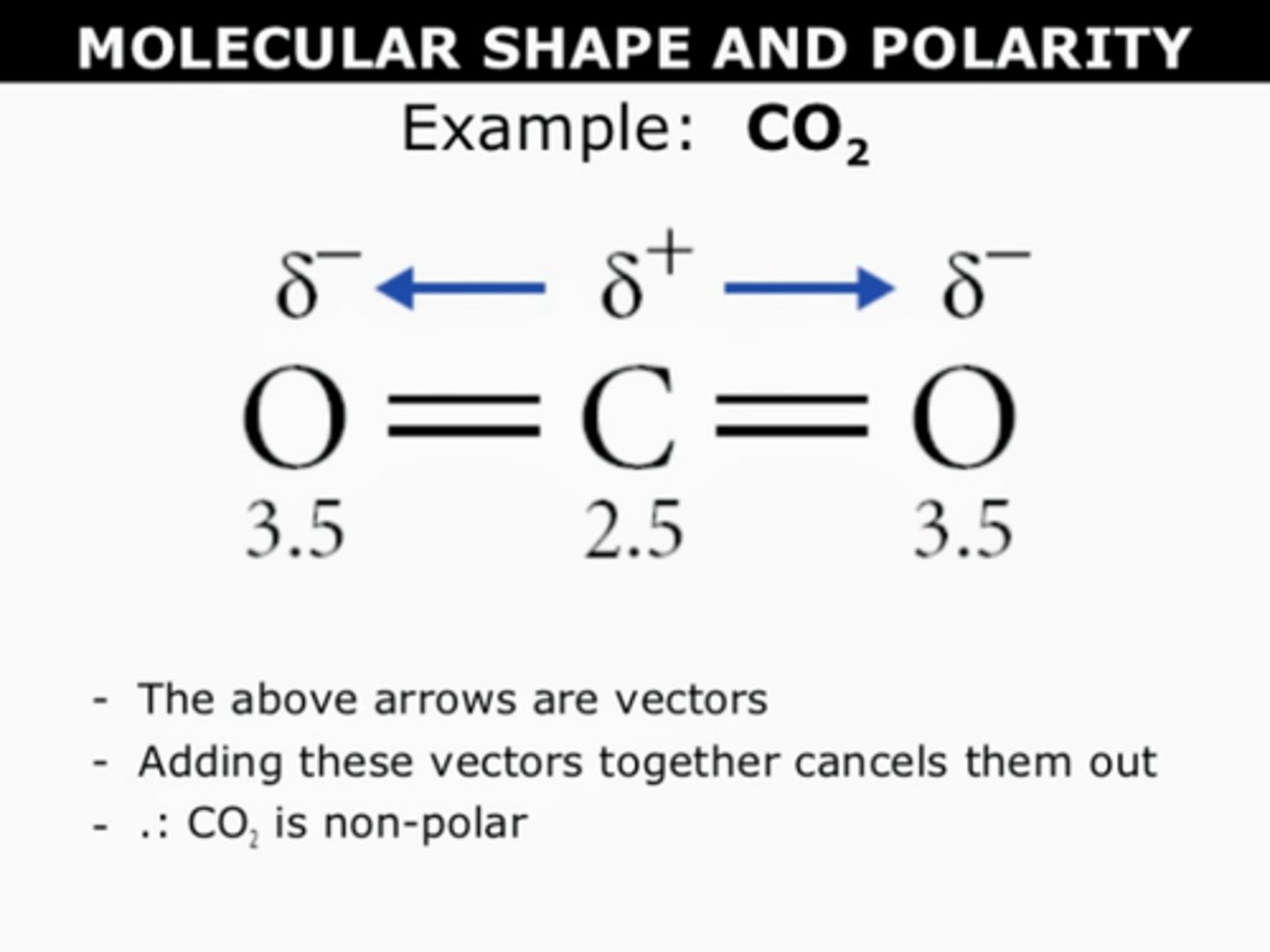
why is CO2 non-polar?
symmetrical
both ends have the same dipole (δ-) = cancels out
polar bonds point away from each other
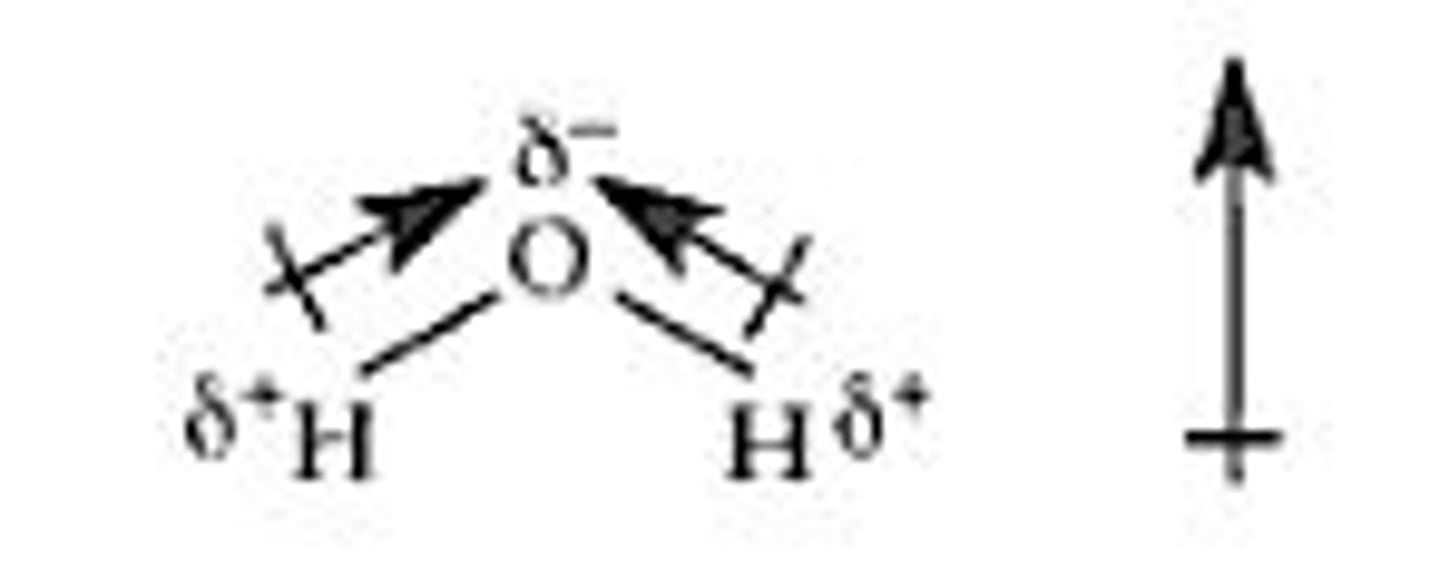
why is H2O polar?
asymmetrical
contains both δ+ and δ- ends = do not cancel out
polar bonds point in the same direction as each other
what do van der Waals' forces consist of? (2)
induced dipole-dipole interactions
permanent dipole-dipole interactions
induced dipole-dipole interactions
1) electron cloud is mobile = uneven distribution of electrons
2) temporary dipole = induces dipoles in neighbouring molecules
2) dipole δ+ and δ- ends ATTRACT each other = form dipole-dipole interactions
induced dipole-dipole interactions TREND
larger molecule → more electrons → stronger dipole-dipole interactions → higher boiling point
permanent dipole-dipole interactions
stronger than induced
1) difference in electronegativity leads to a PERMANENT dipole + polar bonds
2) attraction between δ+ and δ- ends → WEAK IMF formed
define redox reaction
reduction and oxidation occur simultaneously
no net gain/loss of electrons
hydrogen bonds
strongest intermolecular force
3 molecules which form hydrogen bonds
HF
H2O
NH3
why do HF/H2O/NH3 form hydrogen bonds?
difference in electronegativity between H atoms and very electronegative N/F/O
properties of water
ice is less dense than liquid water
H2O molecules are arranged further apart in a lattice structure = floats
unusually high boiling point
due to hydrogen bonds
surface tension
due to strong H bonds on surface
half equations
example: oxidation of magnesium
Mg --> Mg 2+ + 2e -
reducing agent
is oxidised (donates electrons)
reduces other
oxidising agent
is reduced (accepts electrons)
oxidises other
name the 3 molecules which form hydrogen bonds
H2O
HF
NH3
H is not very electronegative
O, F and N are very electronegative
= large difference in electronegativity
questions on boiling point guide e.g. why does water have a higher boiling point than ethane?
1) identify IMFs in molecule
2) compare strengths of IMFs
3) amount of energy required to overcome (more/less)
questions on shapes of molecules + repulsion guide e.g. explain why ammonia has a trigonal pyramidal shape
1) name of molecule's shape
2) describe structure - lone pairs, bonding pairs (+ angle if needed)
3) describe repulsion