covalent bonding in hydrogen, chlorine and hydrogen chloride
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
what is a covalent bond?
when atoms share pairs of electrons.
covalent bonding takes place between?
non-metal elements
the bonds between atoms in a covalent bond are?
strong
example of a simple covalent molecule
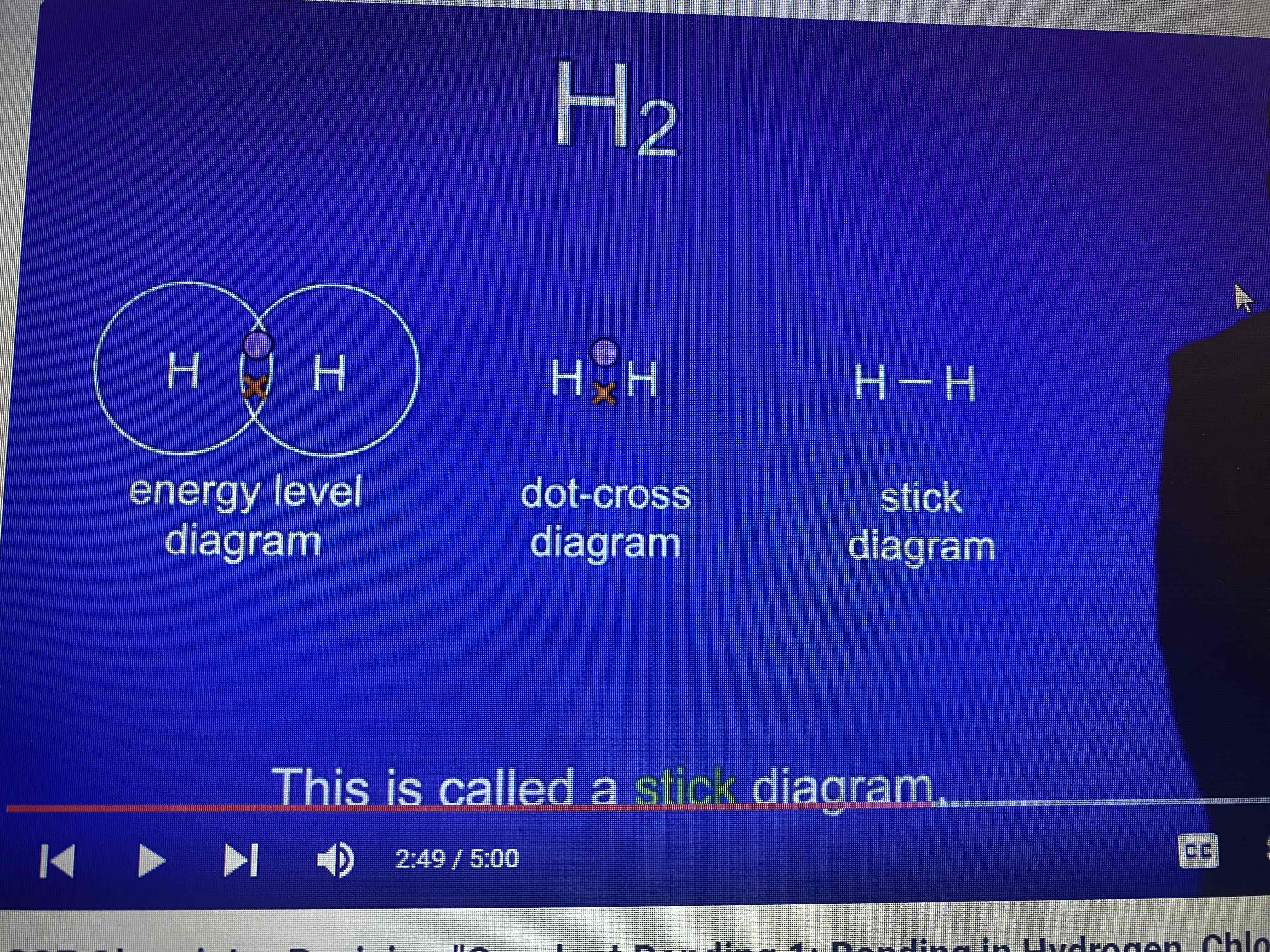
hydrogen molecule H₂
what does H₂ tell us?
two hydrogen atoms bonded together
what is hydrogen?
a non-metal element
hydrogen has only one electron to make a full outer energy level how many more electrons does hydrogen require?
one more electron
how does a hydrogen molecule achieve getting another electron?
by bonding with another hydrogen atom.
the two hydrogen atoms overlap their energy levels and they share their electrons
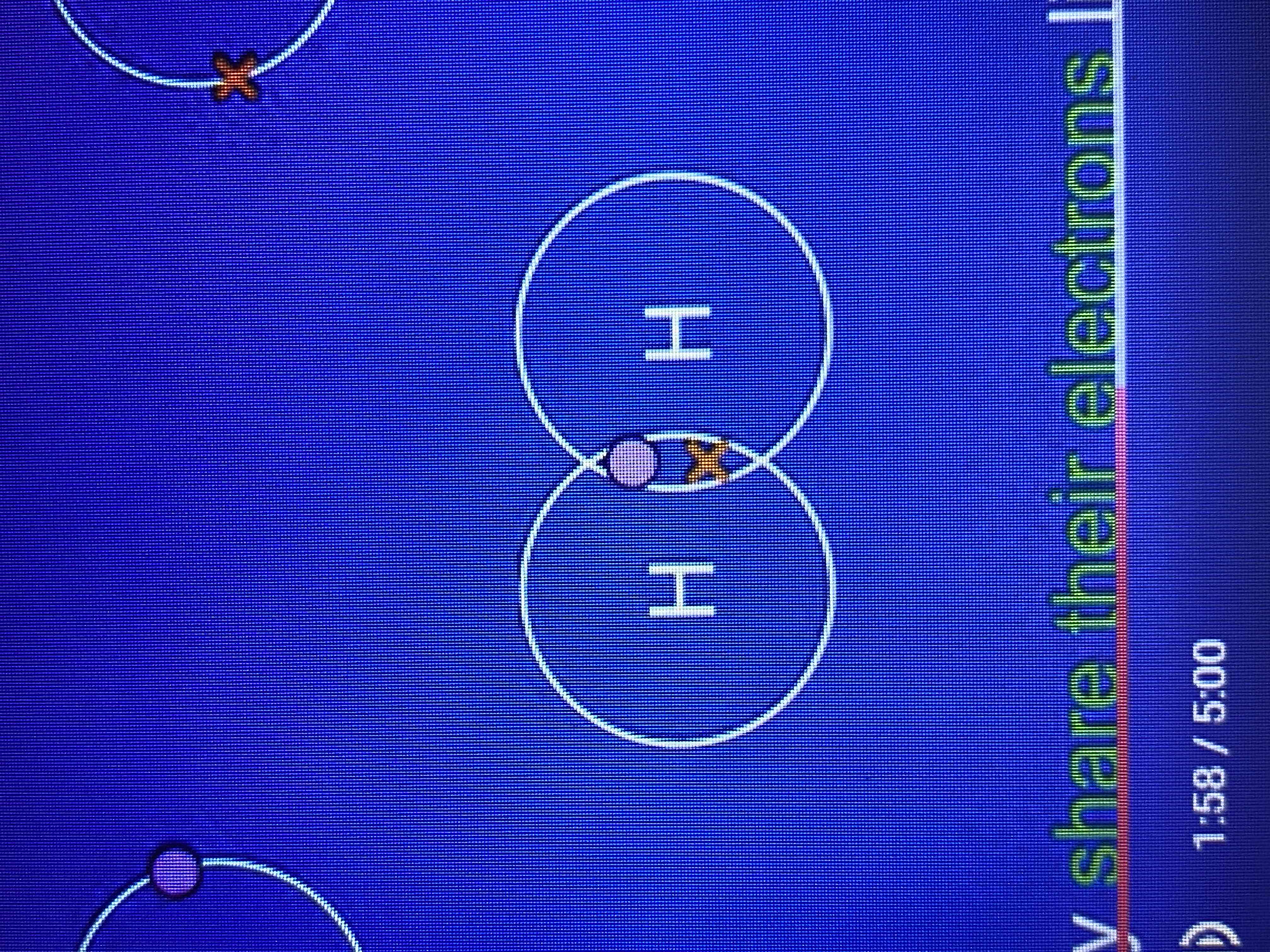
due to both hydrogen atoms having two electrons what have they achieved?
a full outer energy level like a group 0 noble gas.
by sharing a pair of electrons what have the hydrogen atoms formed?
a single covalent bond
ways to represent covalent molecules
an example of a slightly more complicated molecule
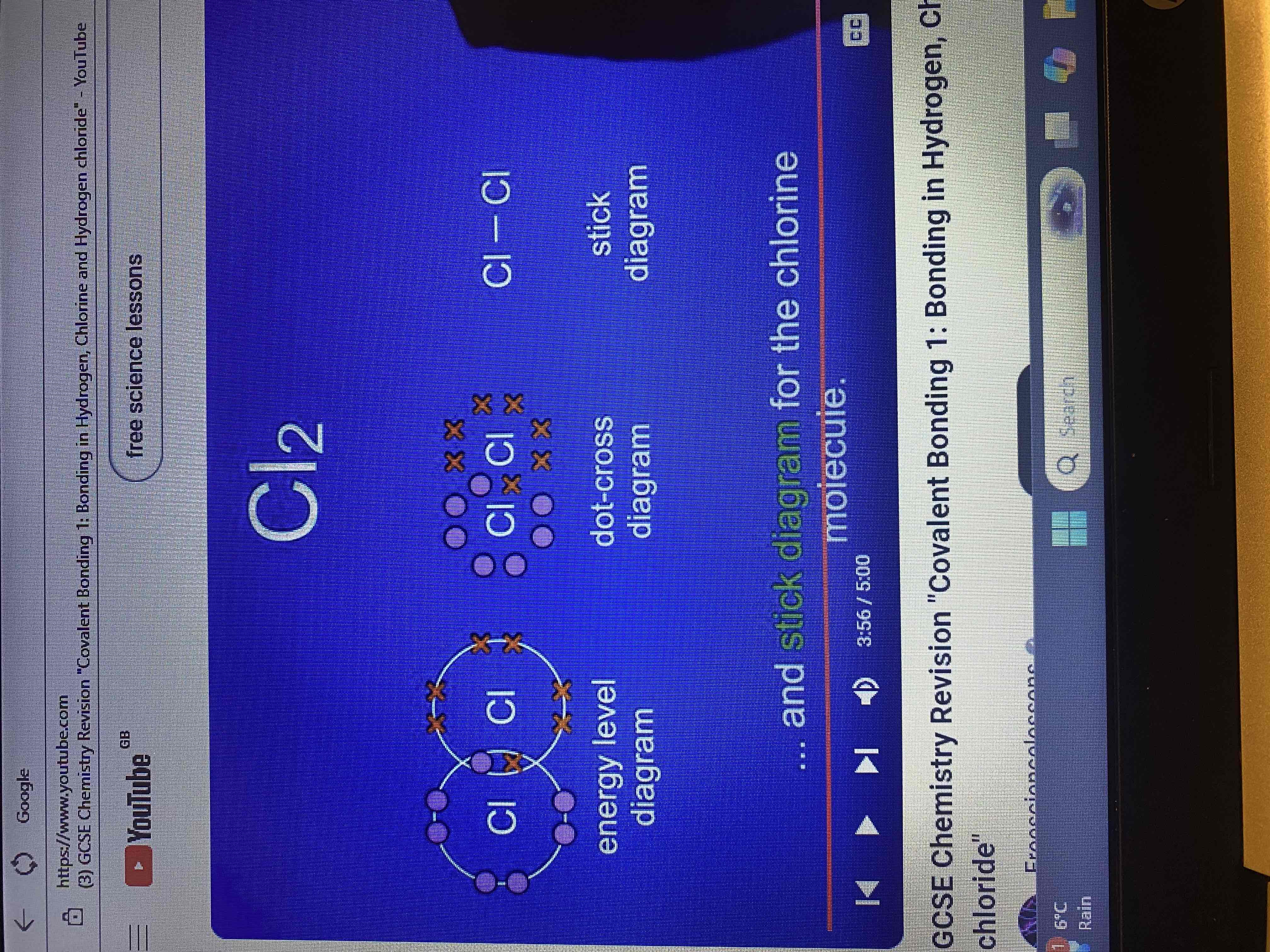
chlorine molecule Cl2
what is only involved in chemical bonding?
outer energy levels
chlorine has 7 electrons in its outer energy level, to achieve a full outer energy level what does it require?
each atom of chlorine requires one more electron.
energy level diagram for chlorine molecule
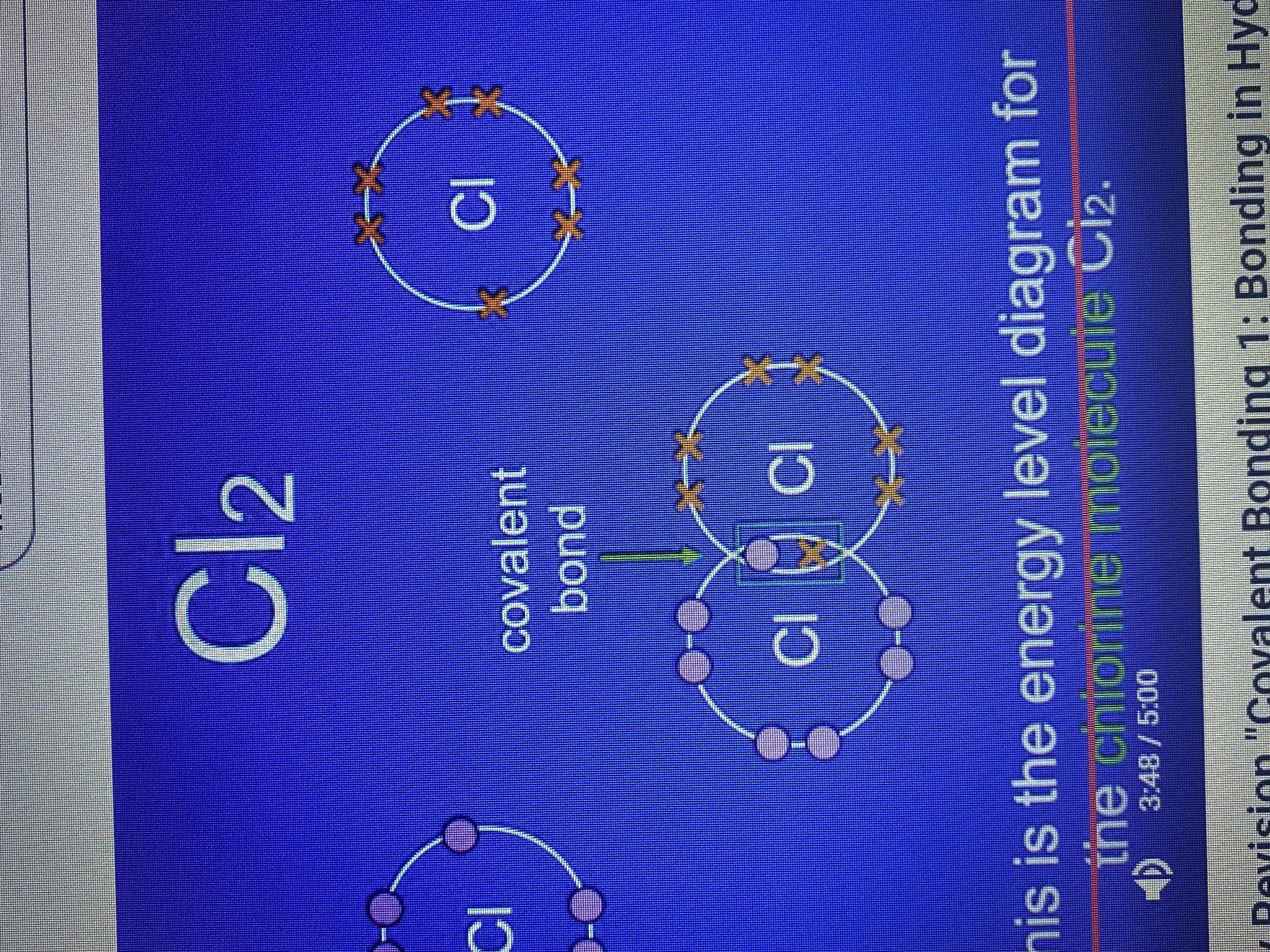
dot a cross diagram and stick diagram for chlorine molecule
example of small covalent molecule
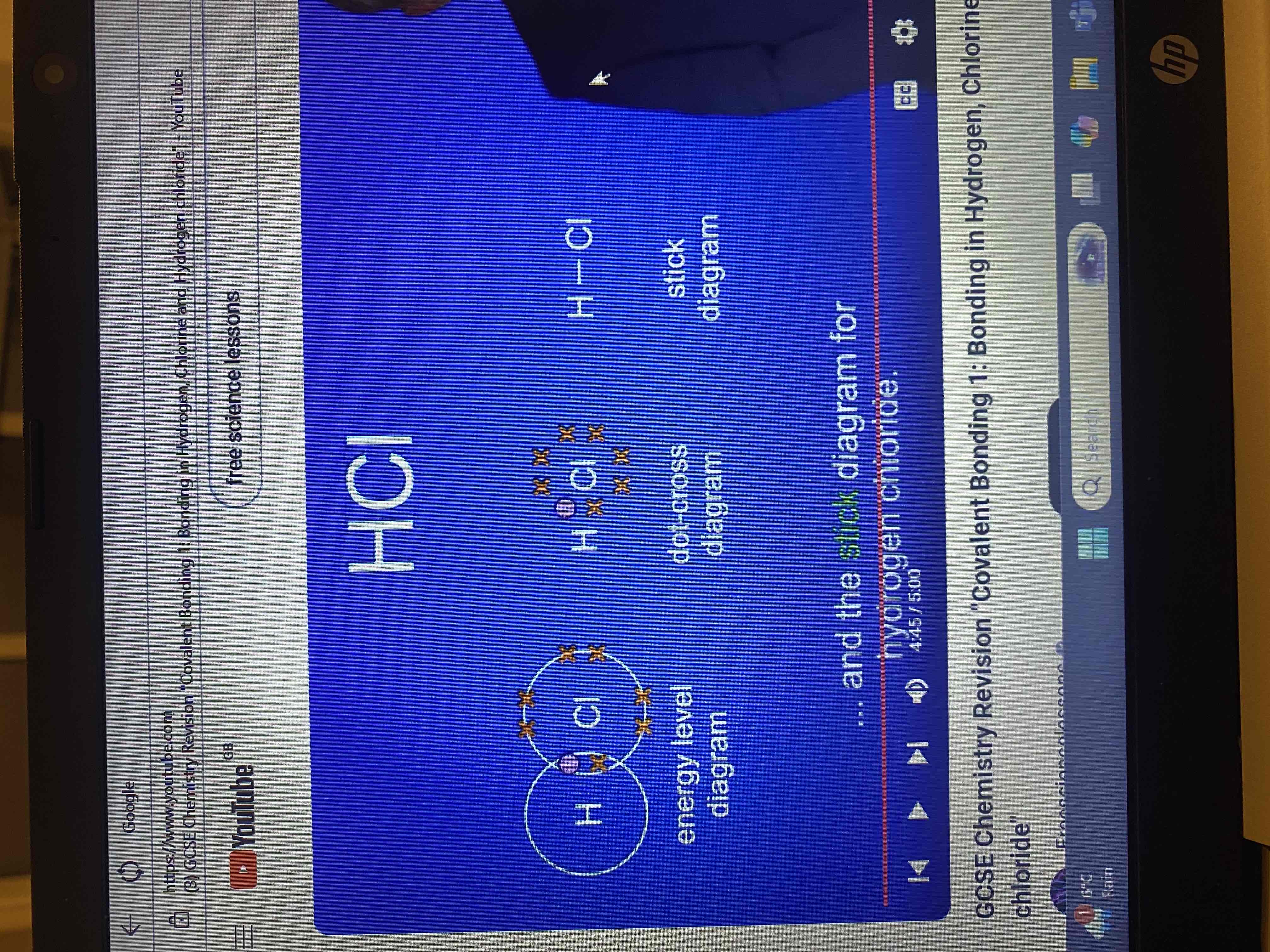
hydrogen chloride HCl
what are both hydrogen and chlorine?
non-metals
a hydrogen atom has one electron and a chlorine atom has seven electrons in its outer energy level what do both atoms require?
one extra electron
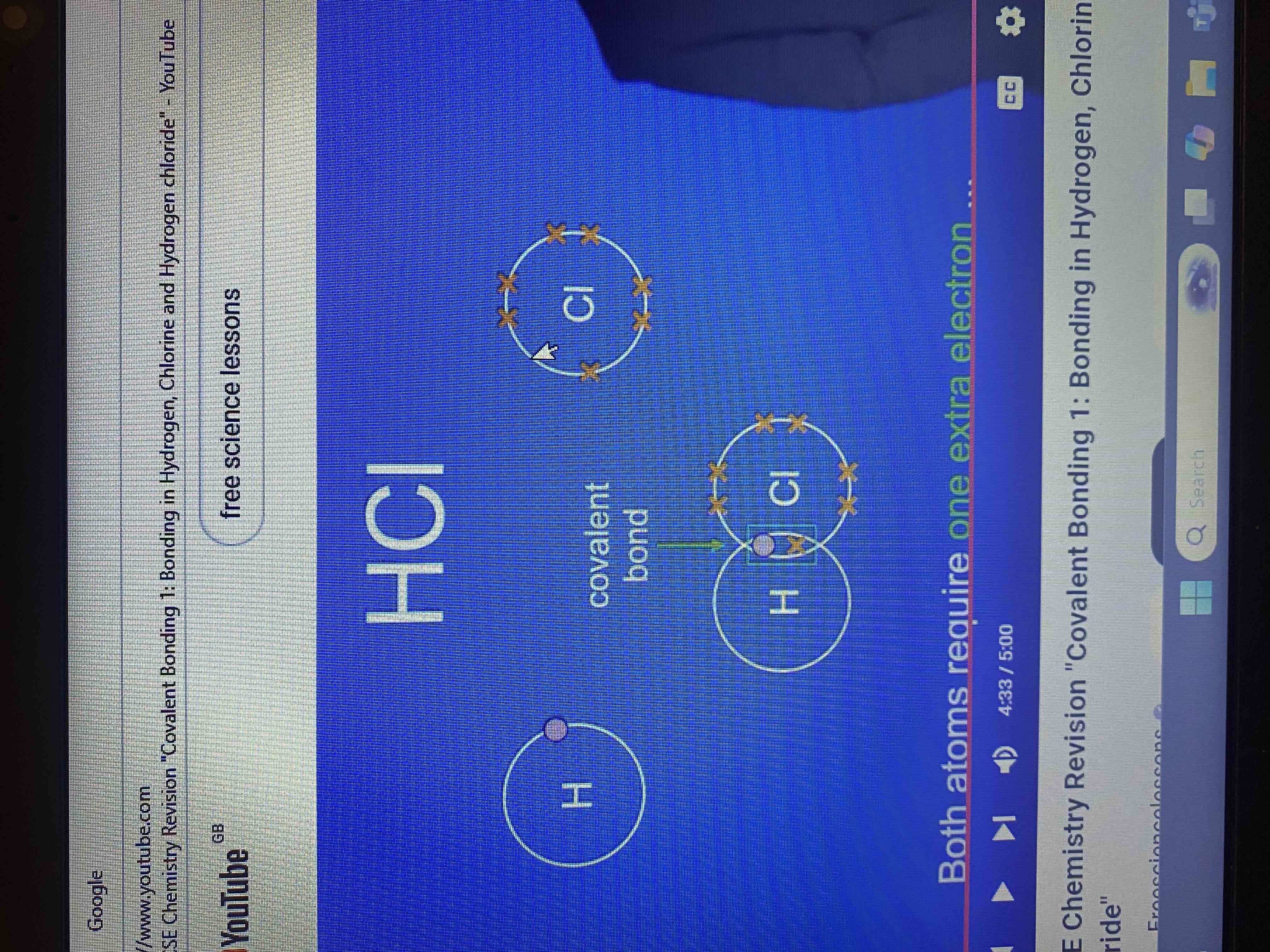
dot and cross diagram and stick diagram for hydrogen chloride