Water potential
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Define solute, solvent and solvation.
Solute: the substance that is dissolved in the solvent.
Solvent: The liquid in which the solute dissolves.
Solvation: The process of surrounding solute particles with solvent particles to form a solution.
It involves the breaking of bonds within the solute and the solvent and the formation of new bonds between the solute and the solvent molecules.
The definition and net movement of water of Hypertonic solution
when it has a higher solute concentration compared to another solution.
there is a greater osmotic pressure, and water tends to move out of cells or organisms into the hypertonic solution.
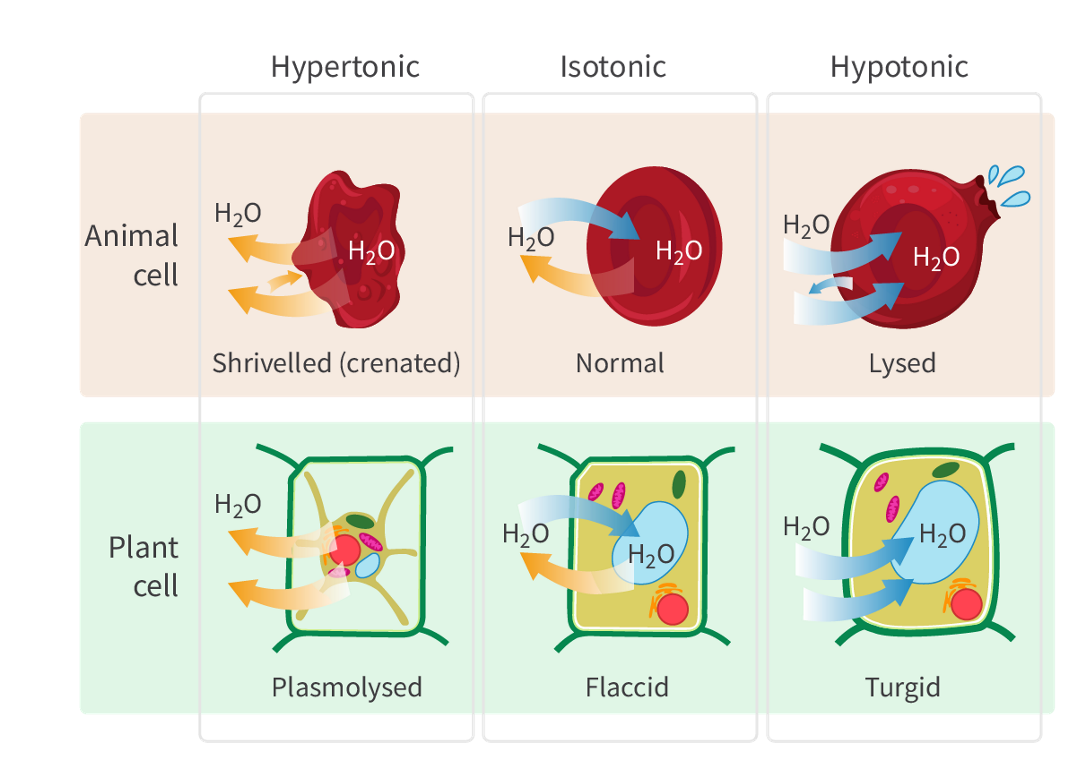
The definition and net movement of water of Isotonic solutions
when it has the same solute concentration as another solution.
there isa dynamic equilibrium
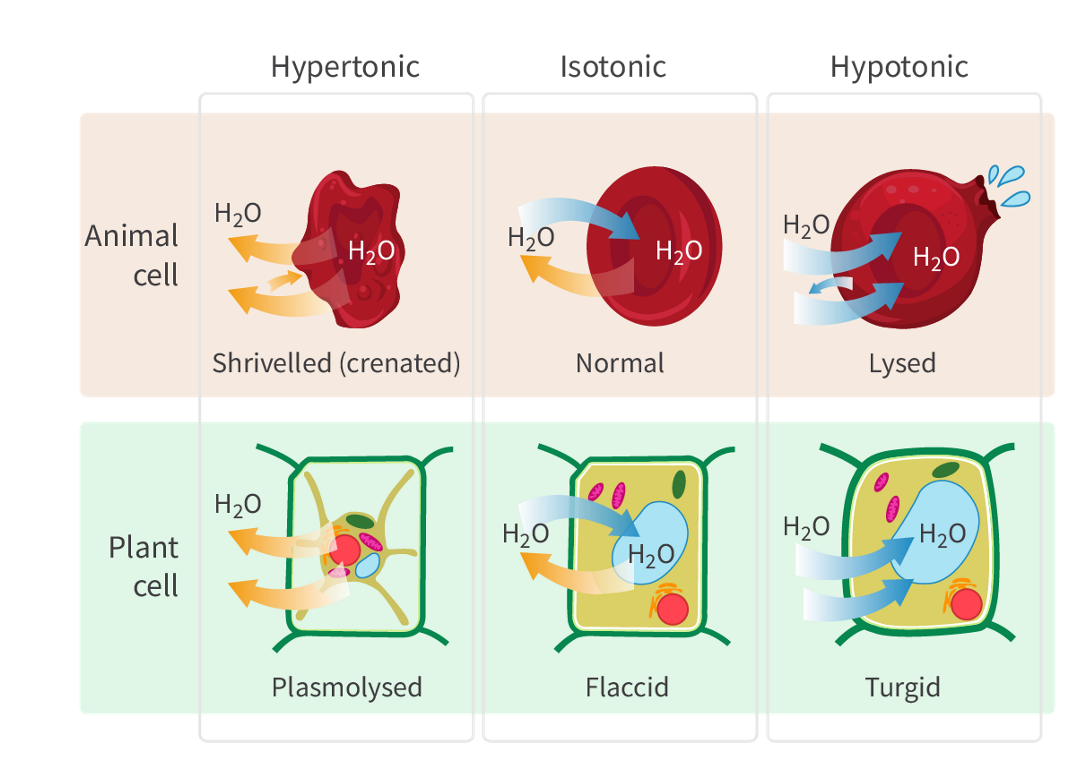
The definition and net movement of water of hypotonic solutions
when it has a lower solute concentration compared to another solution.
there is a lower osmotic pressure, and water tends to move into cells or organisms from the hypotonic solution.
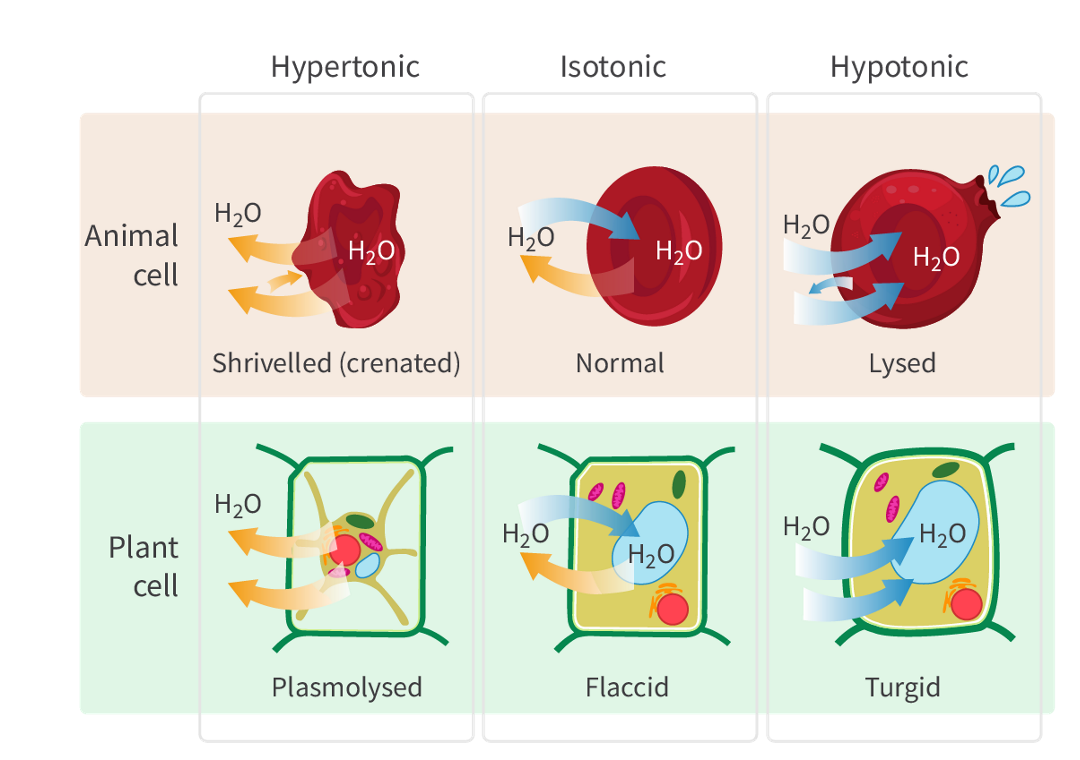
Hypertonic hypotonic and isotonic definitions

Explain why water is able to dissolve charged and polar molecules
If the force of attraction between the ions and water molecules is greater than the forces of attraction between the oppositely charged ions, water has the ability to dissolve the substance.
the slightly positively charged hydrogen atoms of water will be attracted to the negatively charged ions and the slightly negatively charged oxygen atoms will be attracted to the positive ions. Water molecule surrounds the ions creating hydration shells.
the presence of these hydration shells leads to the separation of solute particles and their uniform distribution throughout the solution (dissolution).
How can covalent compounds dissolve in water?
by forming intermolecular interactions with dipolar water molecules
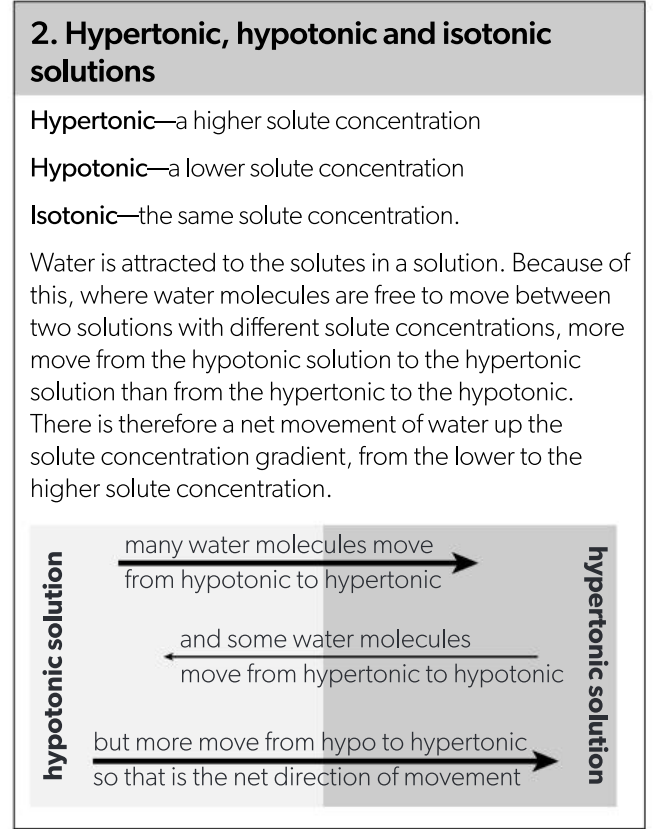
Outline the movement of water in hypertonic and hypotonic solution
Water potential
It is the potential energy of water per unit of volume.
It describes the potential of water to move across a partially permeable membrane.
Unit: Kilo pascals
Symbol: psi (Ψ)
Define osmosis.
a passive form of transport which is the diffusion of water molecules from a region of higher to lower water potential.
Water potential of pure water compared to solutions
pure 20° water at standard atmospheric pressure as a water potential of 0kPa.
any solutions have a water potential of less than 0.
Outline the effects of bathing plant tissues in a hypotonic solution (5 marks)
When the external solution is less concentrated (hypotonic)than the cell solution:
+there is a net inflow of water into the cells by osmosis
+the cell becomes diluted causing the permanent vacuole to swell due to water uptakeand the membrane of the vacuole is pushed against the cytoplasm which in turn presses hard against the cell wall, increasing turgor pressure
the cell is turgid; the pressure that develops (due to the stretching of the cell wall) eventually becomes so great it prevents further uptake of water
the cell wall protects the cell contents from damage due to osmosis but the tissue may be rigid due to the internal pressure
Outline the role of the cell membrane in maintaining the osmotic concentration of cells. [9]
The hydrophobic hydrocarbon chains that form the core of a plasma membrane have low permeability to large molecules and hydrophilic/charged particles, including ions and polar molecules;
membranes function as effective barriers between aqueous solutions;
osmosis is the diffusion of free water molecules from a region where they are more concentrated (low solute concentration) to a region where they are less concentrated (high solute concentration) across a partially permeable membrane;
water can diffuse across the plasma membrane via aquaporins in the membrane and via tiny spaces between the phospholipid molecules;
ions can be pumped across the membrane to increase the osmotic concentration in a cell; water is drawn into a cell by osmosis if the solute concentration inside the cell is higher than outside it;
water cannot be pumped directly across the membrane but changes in ion/solute concentration across the membrane can draw water into a cell from a lower to higher osmotic concentration/from a dilute to concentrated solution;
this process maintains the osmotic concentration of cells; there is net movement of water if the environment of a cell is hypotonic or hypertonic; in an isotonic environment there is dynamic equilibrium rather than no movement of water.
Describe how the osmotic concentration of tissue cells can be determined. [7]
Cut tissue to specific size, e.g. potato chips;
take mass of tissue;
put tissue in a range of different solute concentrations, e.g. sucrose or salt;
leave the tissue for at least 24 hours and reweigh tissue (after first drying tissue);
calculate percentage change in tissue;
plot solute concentration (x-axis) against percentage change in mass (y-axis);
identify the point where there is no change in mass (where line of best fit crosses the x-axis); the point where there is no change in mass indicates isotonicity,
i.e. osmotic concentration of the tissue is the same as the solution, which gives the solute concentration of the tissue.
Outline the contributions of solute potential and pressure potential to the water potential of cells with walls. [7]
The concentration of a solute determines the water potential of a solution;
the pressure within a cell also determines the movement of water, with a higher pressure resulting in greater potential energy and therefore water potential;
water potential is calculated using t ψw = ψs + ψp; where ψw = water potential, ψs = solute potential, and ψp = pressure potential;
adding a solute to a solution lowers the water potential;
pressure potentials are generally positive inside cells; this is because of the movement of water molecules pushing against the cell membrane and cell wall of plant, fungi and prokaryote cells;
negative pressure potentials can occur, inside xylem vessels where sap (mainly water) is being drawn up the plant under tension.
Pressure potential inside cells
pressure potentials are generally positive inside cells; this is because of the movement of water molecules pushing against the cell membrane and cell wall of plant,
negative pressure potentials can occur inside xylem vessels where sap (mainly water) is being drawn up the plant under tension.
Movement of water in a hypotonic solution (with reference to solute and pressure potential)
solute potential of the tissue s more negative than the solute potential of the solution
water moves into the tissue from an area of less negative solute potential to an area of more negative solute potential (to equilibrate the solute potentials of the inside of the tissue and the solution).
the additional water inside the cell causes them to apply an outward pressure (turgor pressure) which pushes the cell membrane against the cell wall
as a result pressure potential is positive because the pressure inside the cell is higher than the pressure outside the cell.
the positive pressure potential can offset the negative solute potential resulting in an equilibrium whereby the water potential of the tissue is equal to the water potential of the solution, maintaining the water balance of the tissue
Movement of water in a hypertonic solution (with reference to solute and pressure potential)
solute potential of the solution is more negative than the solute potential of the tissue
water moves out the tissue from an area of less negative solute potential to an area of more negative solute potential (to equilibrate the solute potentials of the inside of the tissue and the solution).
the decrease in volume of the cell due to waterloos causes a decrease in the pressure in cell relative to the solution which causes it to detach from the cell wall
pressure inside the cell is lower than the pressure outside the cell so pressure potential is negative
What is water potential, solute potential and pressure potential?
water potential is the sum of solute and pressure potential. It determines the speed and extent of water movement.
solute potential: is a measure of the effect of solutes on the potential energy of water molecules in a solution.
negative pressure potential acts as a pulling force or tension and positive pressure potentials act as a pushing force.
What is solute potential and why is it always negative?
it is a measure of the effect of solutes on the potential energy of water molecules in a solution.
When solutes such as salts, sugars, or ions are dissolved in water, they disrupt the regular bonding between water molecules. This disruption reduces the tendency of water molecules to move freely and lowers their potential energy. As a result, the water potential of the solution decreases, meaning it becomes more negative.
The more solute particles present in a solution, the greater the disruption to the water molecules and the lower the water potential becomes. Therefore, solute potential is always expressed as a negative value, reflecting the decrease in potential energy caused by the presence of solutes in the solution.