carbonyl groups (structure/bonding/reactivity)
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
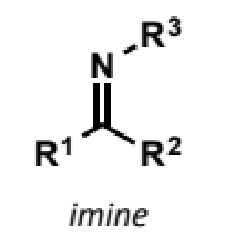
imine
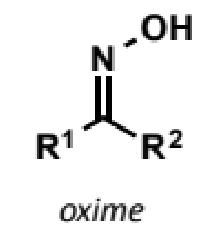
oxime
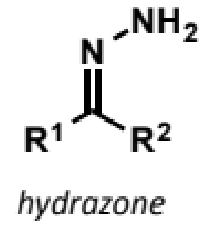
hydrazone
acetal
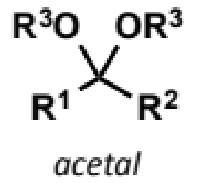
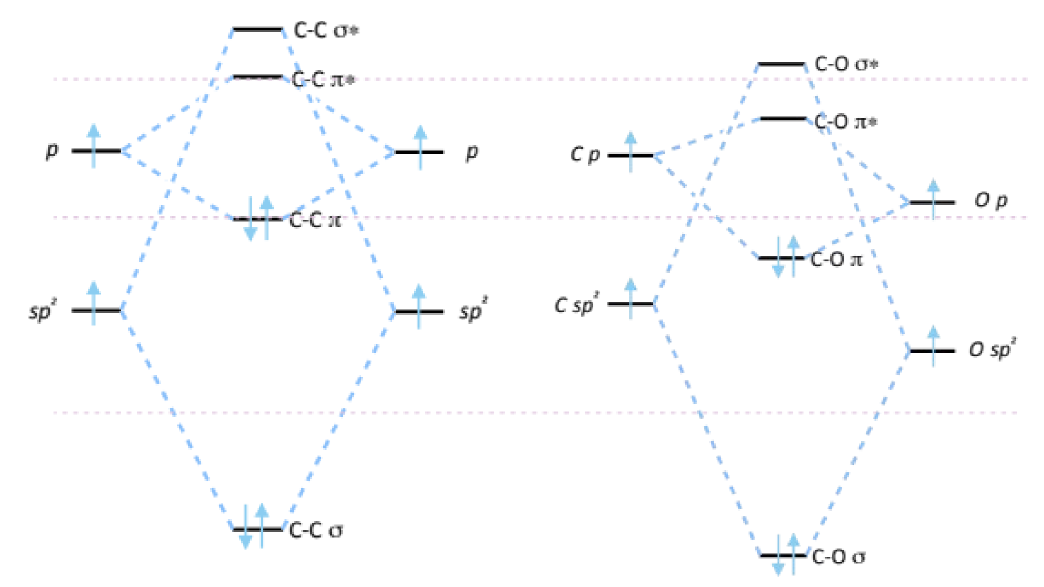
bonding in carbonyl compounds
C-C MO
C-O MO
compare
HOMO for C-O
LUMO for C-O
polarisation?
O orbitals lower in energy (more electronegative)
the C-O MOs are lower in energy - carbonyls are not nucleophilic as nucleophiles have high-lying HOMOs
the HOMOs in C-O are the oxygen lone pairs
the LUMOs are quite low-lying making carbonyls electrophilic
the π orbital is polarised towards O as O is more electronegative. this means the π MO is closer in energy to O than C and the electrons in this bond lie closer to O.
the π* orbital lies closer in energy to C
bond strength order for single + double C-C + C-O
C-C < C=C < C-O < C=O
C=O more than twice as strong as C-O
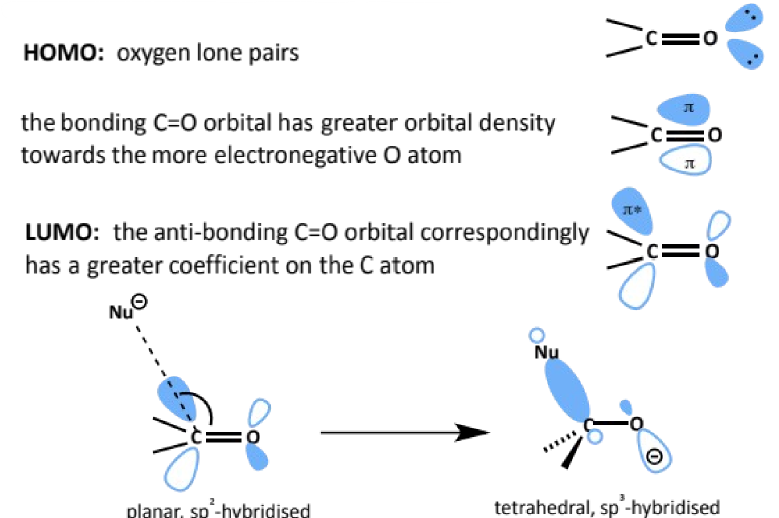
why are carbonyls reactive towards nucleophiles
where does the nucleophile attack
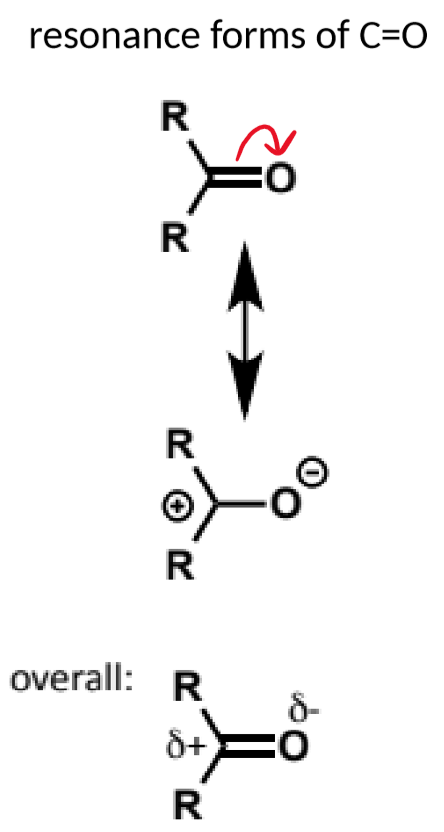
the C=O bond is strong but polarised
nucleophilic species are attracted to the partially positive carbon where they can attack the large lobe of the low-lying LUMO (C=O π*)
the bonding C=O orbital has greater orbital density towards the more electronegative O atom
the antibonding C=O orbital correspondingly has a greater coefficient on the C atom
resonance forms of C=O
water as a nucleophile
which compounds does it react with
what does it form
equilibrium?
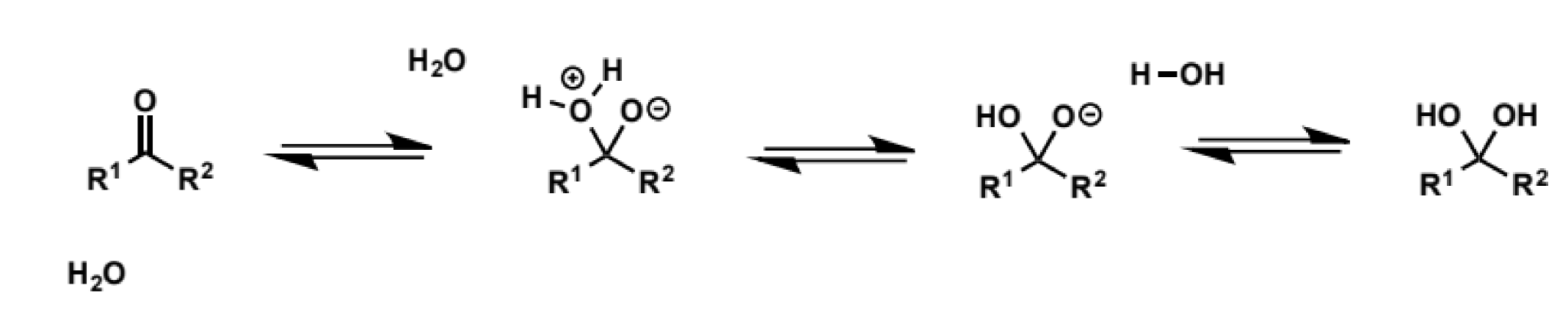
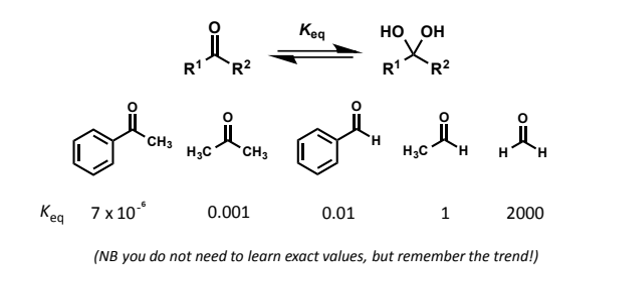
for aldehydes + ketones
can add itself to carbonyls to generate hydrates or 1,1-diols
the reactions are reversible and generally the equilibrium lies to the side of the carbonyl compound as a strong C=O bond must be broken - the hydrates are “disfavoured enthalpically and entropically”
draw the mechanism for ketone + water
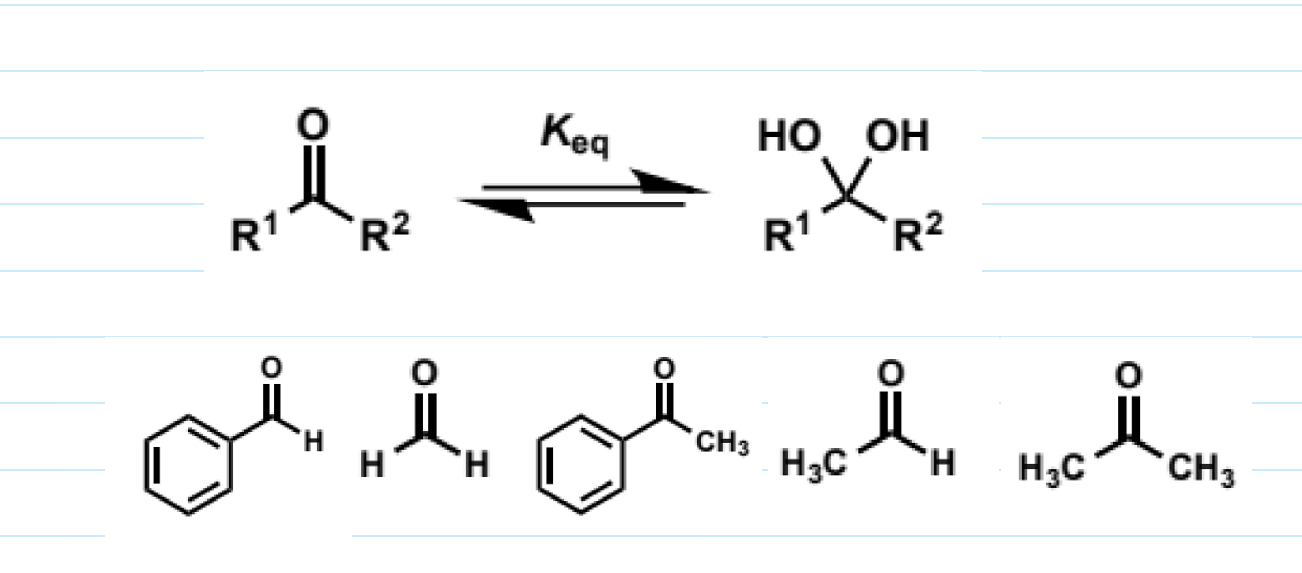
trend in this (hydrate equilibrium)
why?
steric component - larger substituents than H disfavour going to sp3 (which they need to to go from 2 single bonds 1 double bond to 4 single bonds) + it is harder for water to attack
electronic component - stabilisation of carbonyl by hyperconjugation/conjugation lost on going to sp3
these effects mirror those seen in reactivity
aldehydes are generally more reactive than ketones
alkyl substituted carbonyls more reactive than aryl
large substituents
so formaldehyde is best as no steric hindrance and no stabiising EDGs
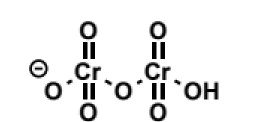
structure of dichromate anion
oxidation of secondary alcohols
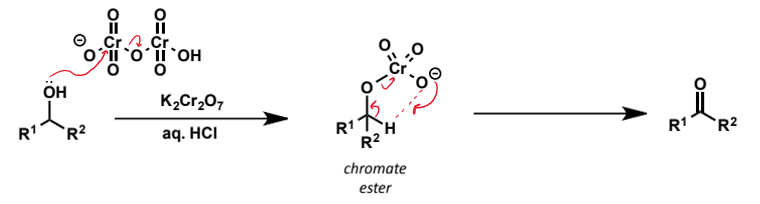
oxidation of primary alcohols
oxidation does not stop at the aldehyde but instead form acids via the hydrate
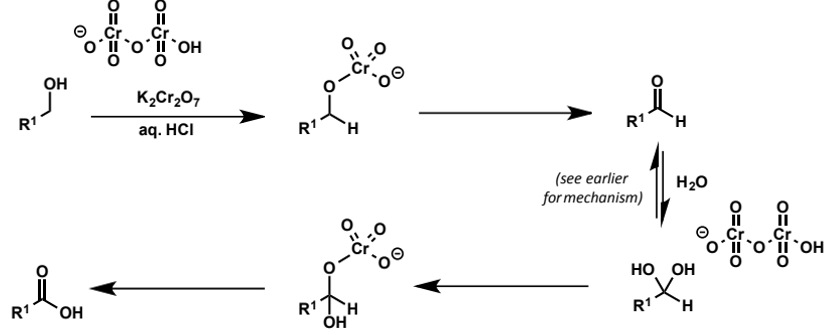
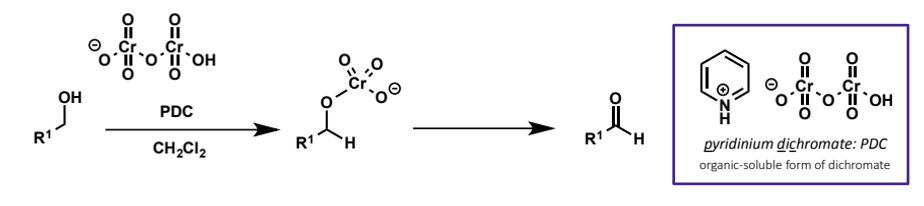
how to get only aldehyde from primary alcohol
need to avoid presence of water so that hydrate and hence carboxylic acid cannot form
do this by using pyridinium dichromate, a form of the dichromate anion which is soluble in organic solvents
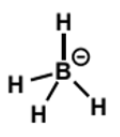
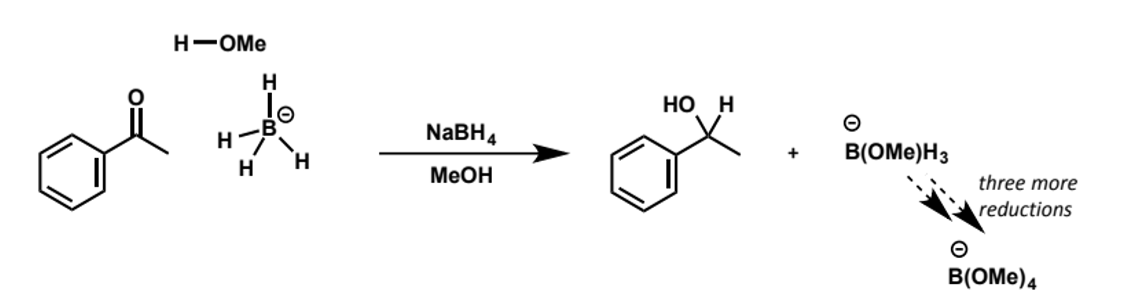
sodium borohydride as a reducing agent
the boron is not nucleophilic - all the electrons are in B-H bonds
the reacting HOMO is a B-H bond
all 4 hydrides can be delivered from -BH4
reduction
nucleophile
solvent
mechanism
reduction with sodium borohydride is carried out in protic solvents, often alcoholic eg MeOH, EtOH, giving the alcohol directly
reducing agent in cells/in nature
NADH - the reduced form of NAD+ (nicotinamide adenine dinucleotide)
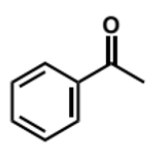
alternative reducing agent to sodium borohydride
conditions?
lithium aluminium hydride, LiAlH4
much more reactive and must be used in aprotic solvents as it has a very violent reaction with water/alcohols
reduction with LiAlH4
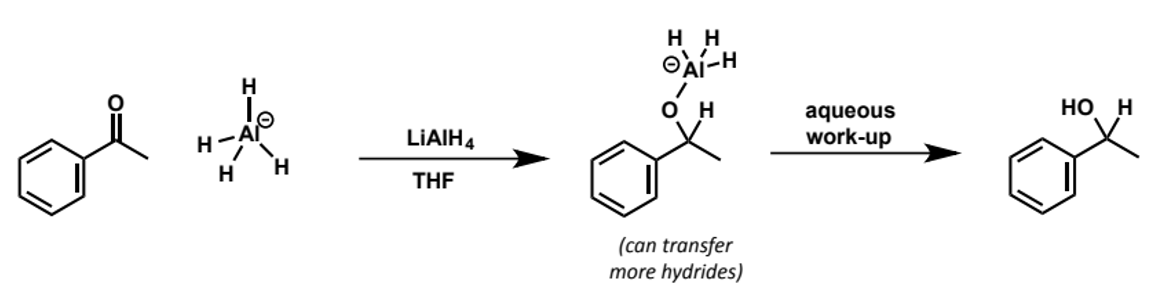
why are both reducing agents used
lithium aluminium hydride reduces many more functional groups than sodium borohydride so the reaction outcomes are different
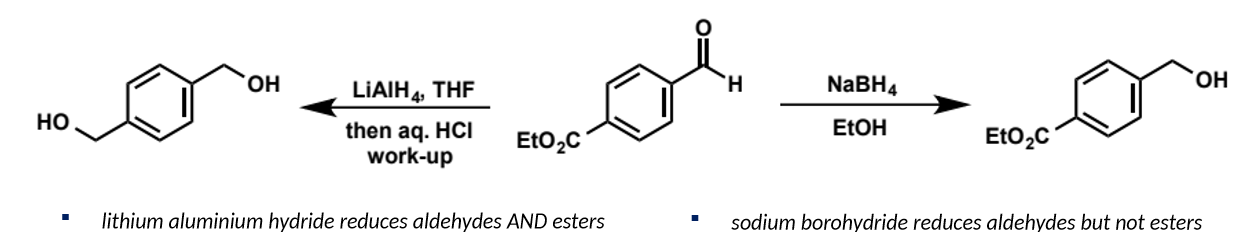
carboxyl derviative
C/H substituent of ketone/aldehyde replaced by a heteroatom group
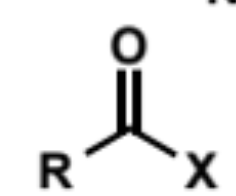
addition of heteroatom Nu to carboxyl derivative
the heteroatom on the carboxyl is a potential leaving group so when the tetrahedral intermediate is formed it can either reform the starting materials or form a different product (substitution)
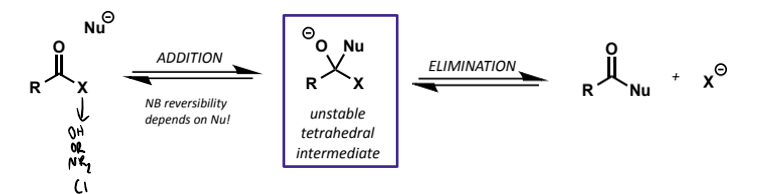
mechanism of Nu substitution name and rules
known as addition/elimination
always has 2 steps with tetrahedral intermediate being formed - never draw as SN2
Fischer esterification and its speed
form by condensation of carboxylic acids with alcohols to give esters and wwater
reaction is slow on its own but catalysed by acid
Fischer esterification mechanism for butanoic acid and methanol
don’t forget sulfuric acid catalyst
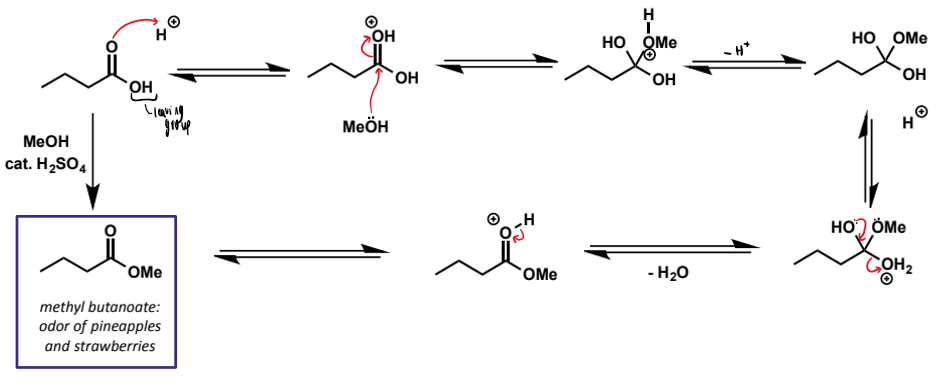
equilibrium for Fischer esterification
reaction is an equilibrium and roughly thermoneutral due to similar structure and bonding of carboxylic acids and esters
get a mixture unless equilibrium shifted by
using the alcohol in large excess (suitable for volatile alcohols like MeOH, EtOH)
or driving off the water eg by azeotropic distillation
polyesters
condensation polymerisation of carb acs and alcs can be used to make polyesters
the high temperatures of the industrial process drive off the water by-product as steam, shifting the equilibrium