Chemical Chirality: Final Exam
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
_____ can be used to reduce carbonyls to alcohols
borohydrides (BH4-)
how would your calculated equivalents of ephedrine be affected if we didn't use MgSO4
equivalents would be higher since the solution will not be dried
sodium borohydride reduction (racemic reduction) procedure
1. MBF and methanol added
2. cool to 0 degrees
3. add NaBH4
4. warm reaction for 1 hr
5. quench with HCl
6. rinse RBF with CH2Cl2 in sep funnel
7. BOTTOM layer is organic (methyl mandelate) TOP layer H2O
8. organic layer dried with MgSO4
9. Rotovap
resolution of mandelic acid (chiral resolution) procedure
1. add ephedrine hydrochloride to NaOH in sep funnel
2. extract with ethyl acetate, BOTTOM = water, TOP = ethyl acetate and ephedrine
3. dry ethyl acetate layers with MgSO4
4. weigh out mandelic acid equally and clean w ethanol
5. heat ethanol to dissolve
6. allow to cool
in order to isolate mandelic acid...
add acid to mandelate-amine complex to form mandelic acid and ephedrine hydrochloride
- extraction: amine salt in aqueous and acid in organic phase
why are many extractions in ethyl acetate necessary for mandelic acid?
mandelic acid is more soluble in ethyl acetate than water, but still very soluble in water
- use a small volume of aqueous acid solution
purification of chiral mandelic acid procedure
1. one does suction filtration and other does extractions
2. suction filtration = add ethanol to the crystals and cool in ice bath. allow to cool and observe crystals.
3. extraction = rotovap filtrate
4. add HCl and extract mandelic acid with ethyl acetate
5. dry with MgSO4 and rotovap ethyl acetate
6. dissolve in ethanol and get polarimetry
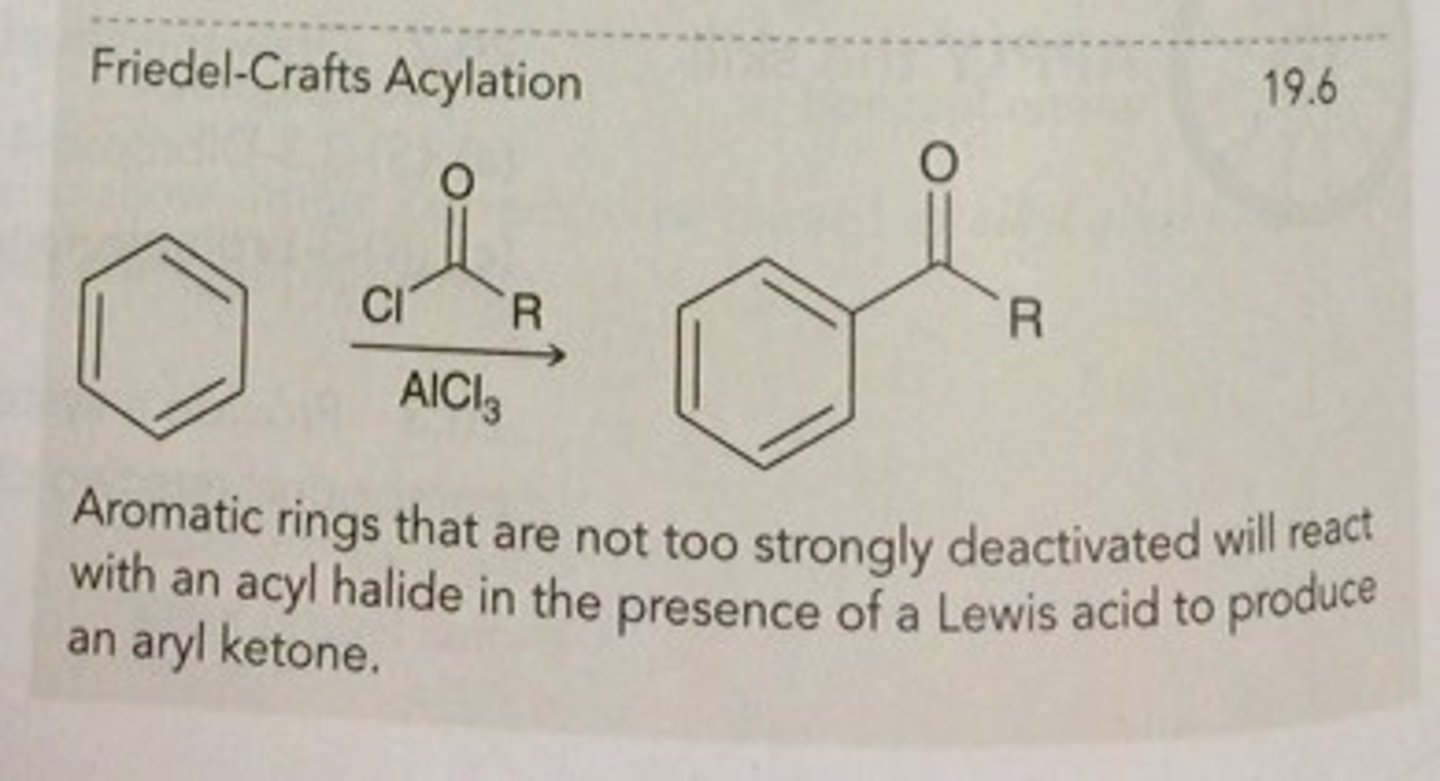
Friedal-Crafts Acylation
electrophilic aromatic substitution mechanism to generate acetylferrocene
enantioselective reduction procedure
1. use a drying tube prepare condensor
2. add THF and tartaric acid
3. wait two hours
4. allow reaction to cool
4. add MBF and continue to stir
5. add HCl and ethyl acetate to separate
6. extract aqueous layer and dry ORGANIC layer
enzymatic reduction procedure pt 2
1. add ethyl acetate to yeast
2. suction filtration and add celite with ethyl acetate
3. transfer to sep funnel and isolate organic (top) and aqueous layers
4. dry organic layers
5. rotovap until you see an oil
synthesis of acetylferrocene procedure
1. combine ferrocene, acetic anhydride, and phosphoric acid
2. gently stir and put in ice water bath
3. neutralize with NaOH
4. suction filtration
why do we increase the temperature in the tartaric acid reaction?
it makes it more selective...thermodynamic barrier for stereoselective pathway must be overcome
if two compounds differ in polarity...separate w....
column chromotagraphy
if one compound is charged and the other neutral...separate with....
extraction
if there are no differences in the polarity or charge of the compound...separate with...
recrystallization
Still learning (103)
You've started learning these terms. Keep it up!