chem lab activity 6
1/25
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
26 Terms
nuclear magnetic resonance spectroscopy (NMR)
measures the magnetic field around all the hydrogen atoms in a molecule to give proximity to a functional group & proximity to other hydrogens, important for structure determination
infrared spectroscopy (IR)
studies the different frequencies of infrared light that a molecule absorbs to indicate the functional groups present
3500 cycle/cm
O-H bonds present
1710 cycles/cm
C=O in ketones & aldehydes present
used less often
NMR & IR
Lucas test
based on the fact that lower molecular weight alcohols are soluble in the solution, which helps the alcohol react with the reagent to form alkyl chlorides which are no longer soluble in the mixture. This creates a cloudiness and turbidity as more of the alkyl chloride is formed
what is Lucas reagent made of?
zinc chloride & hydrochloric acid
immediate reaction w/ Lucas reagent
tertiary alcohol
reaction within 5 minutes of mixing with Lucas reagent
secondary alcohol
no reaction with Lucas reagent
primary alcohol
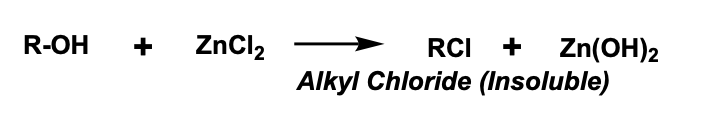
Lucas test reaction
Lucas test on
1-pentanol, 2-pentanol, 2-methyl-2-butanol, and 3-pentanone
iodoform test
If a methyl ketone is present it will react with iodine in a basic solution in order to form an iodoform which is a yellow crystalline solid.
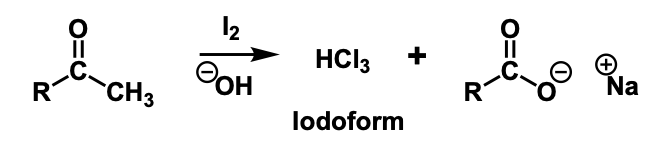
iodoform reaction
iodoform test on
2-pentanone and 3-pentanone
Tollen’s test
using reagent in the presence of an aldehyde there will be a formation of a silver mirror in the reaction vessel. This is caused by an oxidation of the aldehyde to a carboxylic acid and the silver ions are reduced to metallic silver which creates the silver mirror.
what is Tollen’s test unique to
aldehydes
what is Tollen’s reagent made of?
silver nitrate & ammonia
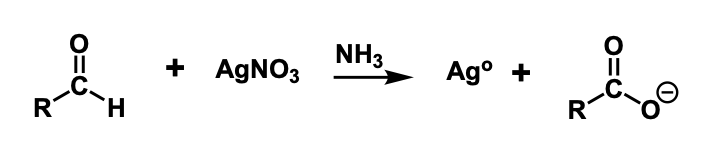
Tollen’s reaction
Tollens test on
benzaldehyde
2,4-DNPH test
When an aldehyde or ketone is treated with 2,4-DNPH, a crystalline precipitate with a unique color will form.
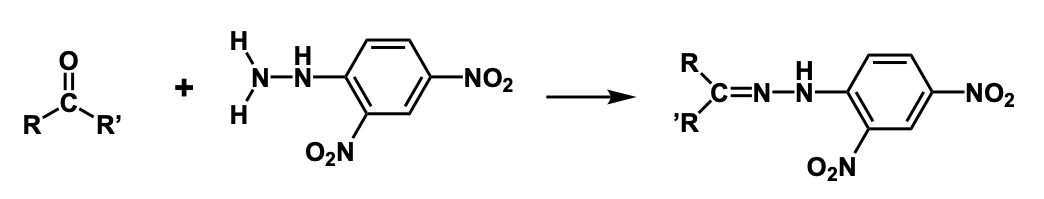
2,4-DNPH reaction
2,4-DNPH test on
3-pentanol and 3-pentanone
hydroxamate test
results in the formation of a colored complex when an ester is heated with hydroxylamine, followed by the addition of ferric (Fe3+) ions
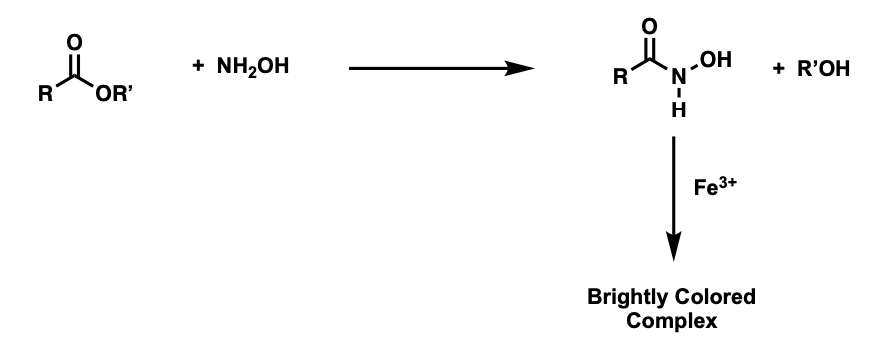
hydroxamate reaction
hydroxomate test on
Aspirin and Salicylic Acid