Practical 2: Protein analysis
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
What is Spectrophotometry
It is a qualitative method for studying matter and is based on the absorption of light by a substance
Lamberts Law
States that the absorbance of light passing through a medium is directly proportional to the absorbing species' concentration and the light's path length.
Beers Law
Relates the absorption of light to the properties of the material through which the light is traveling, stating that absorbance is directly proportional to concentration and the path length of the sample.
what is light
form of Electromagnetic Radiation
Properties of Light
Light waves consist of perpendicular, oscillating electric and magnetic fields
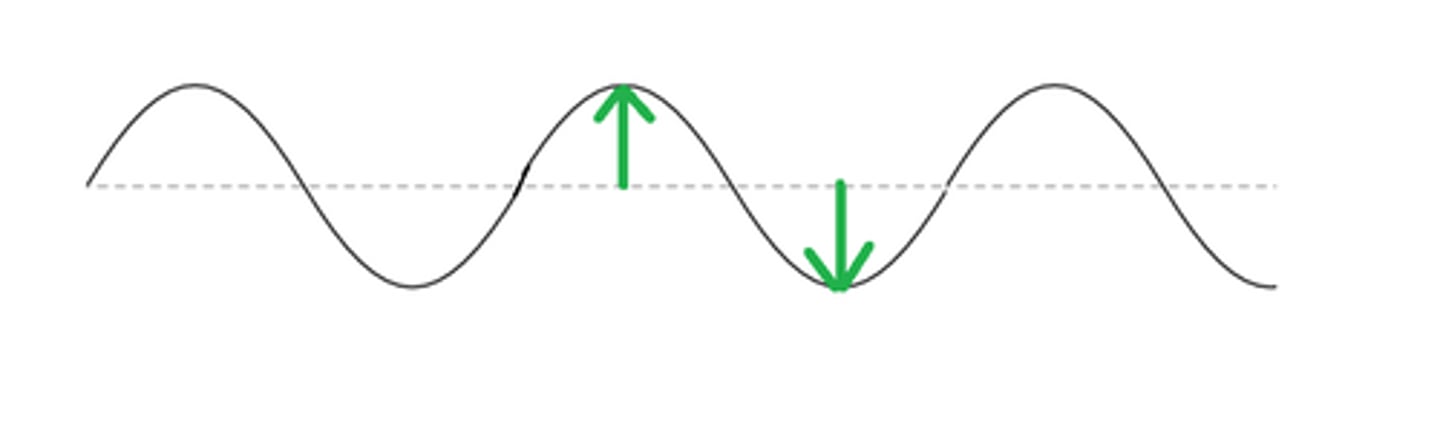
Amplitude (γ)
height of the waves electric vector
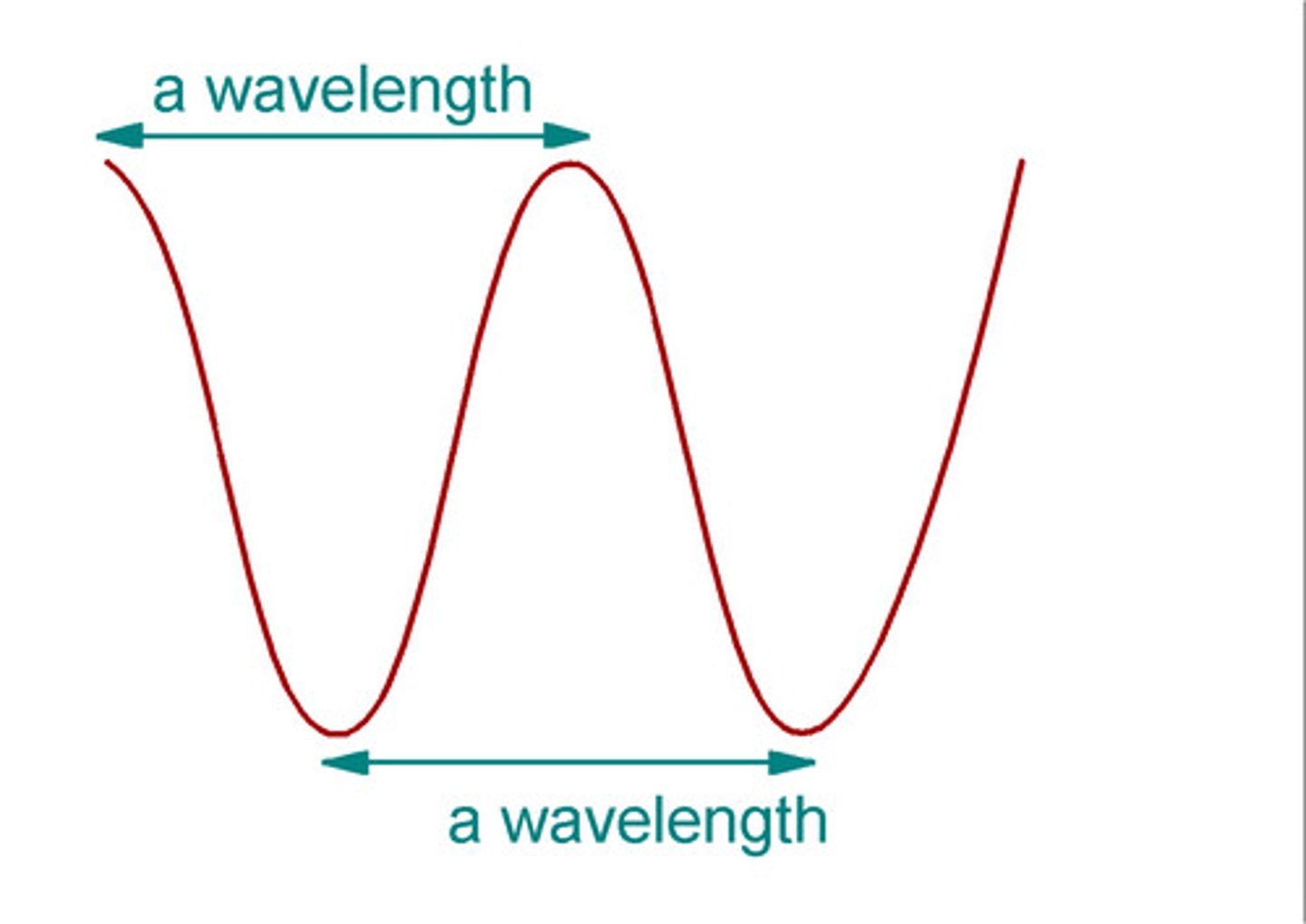
Wavelength (λ)
distance from peak to peak
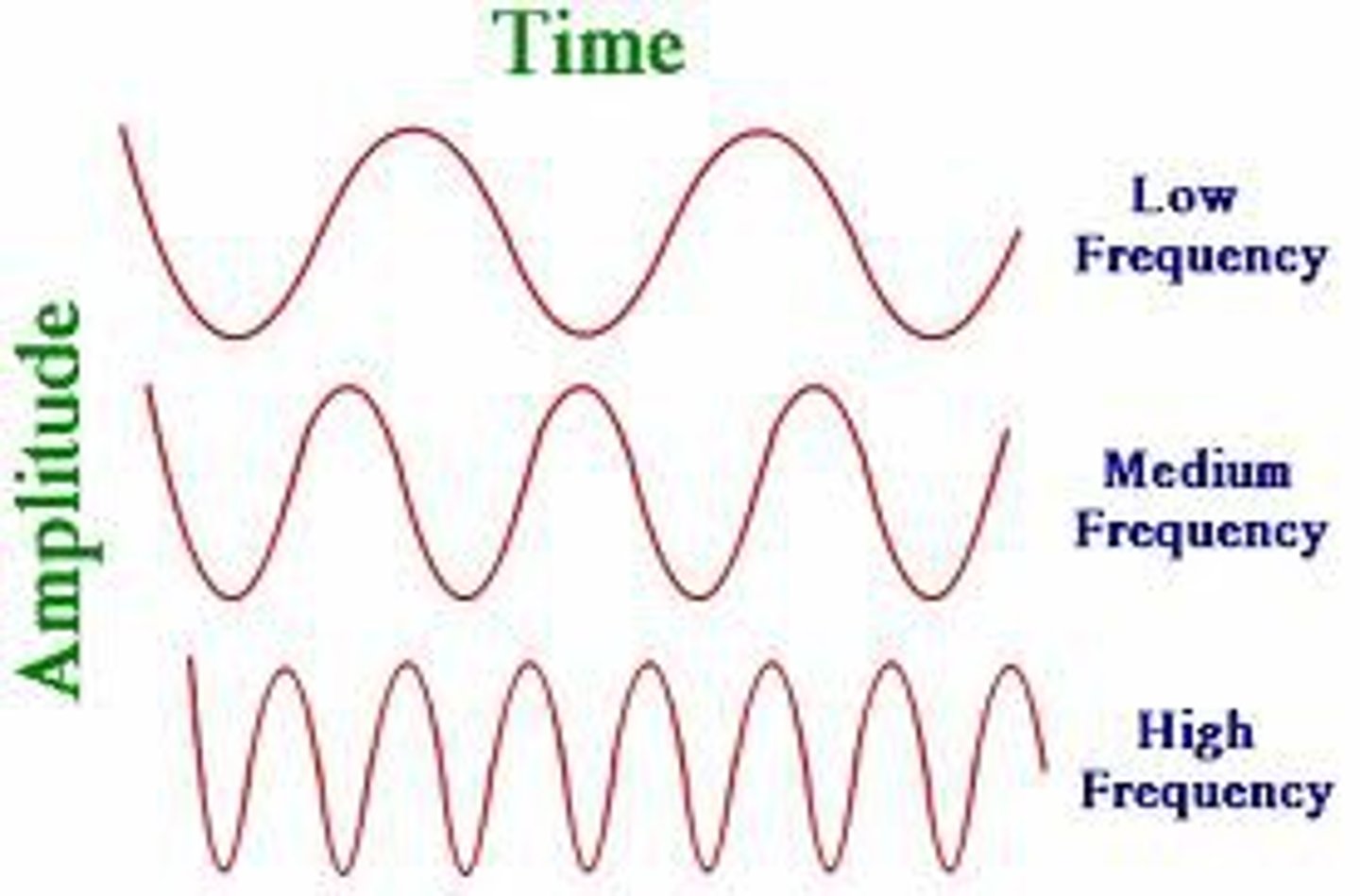
Frequency (f)
number of complete oscillations that the waves makes each second
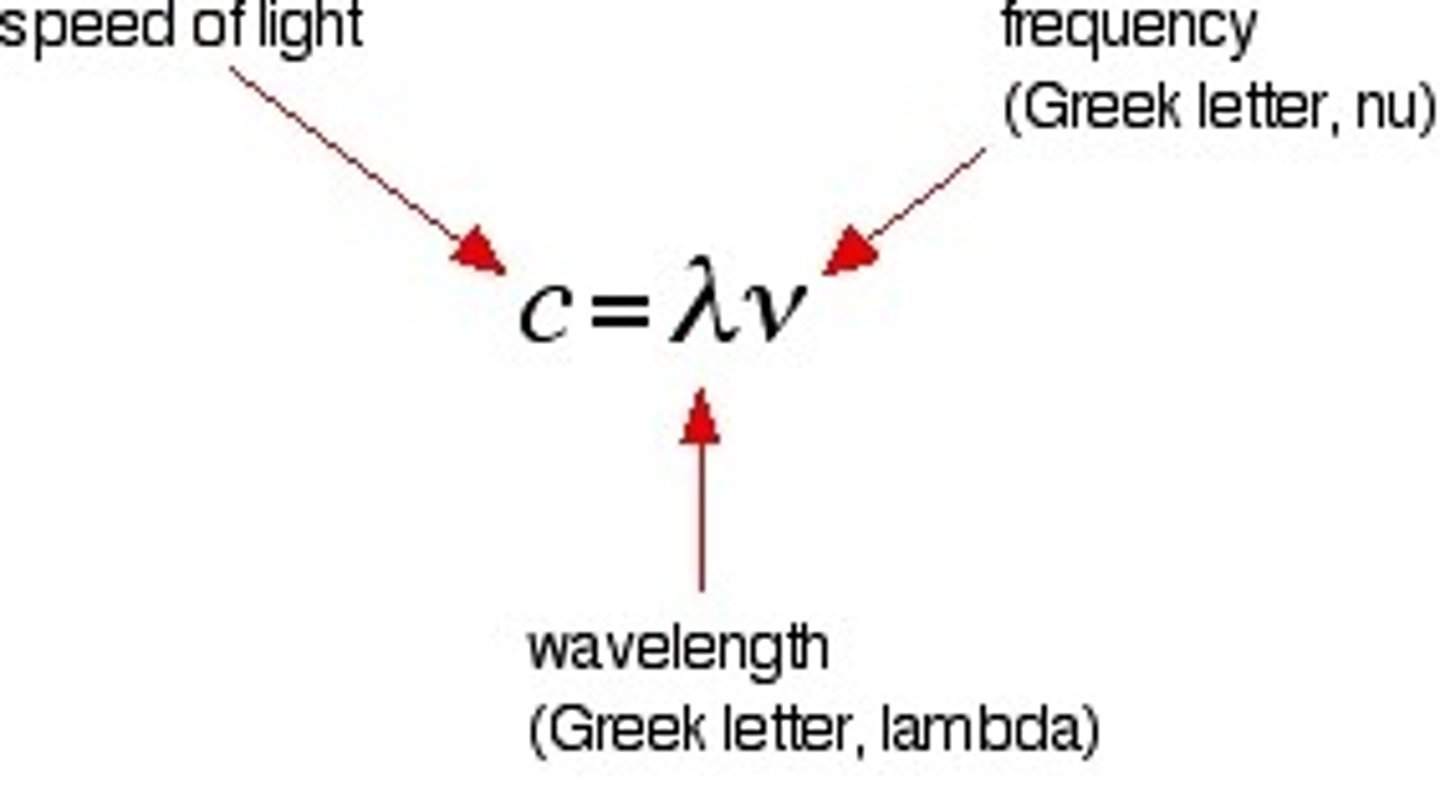
eq of frequency
what are photons
particle aspect of light
energy described in light
The energy of one particle of light (photon) is proportional to its frequency
E = h*f
as frequency (f) increases, energy (E) of light increases
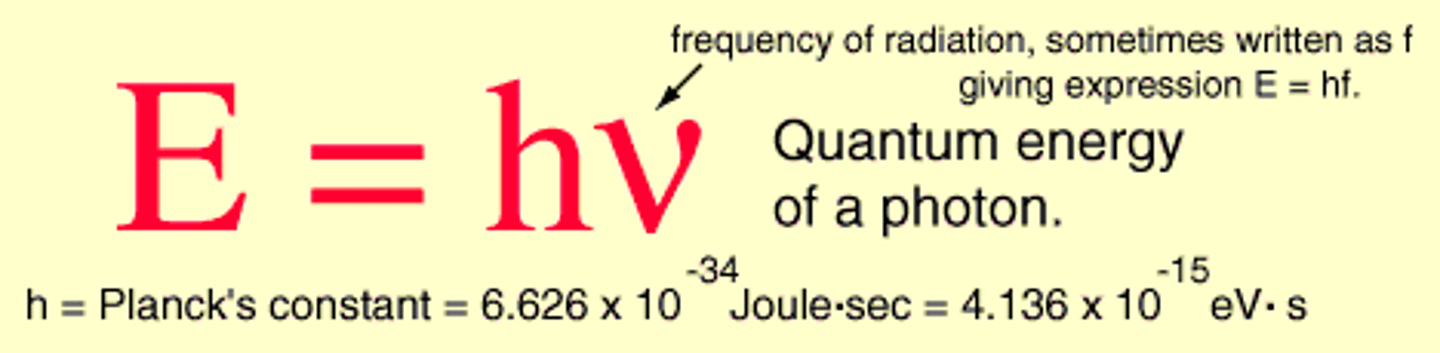
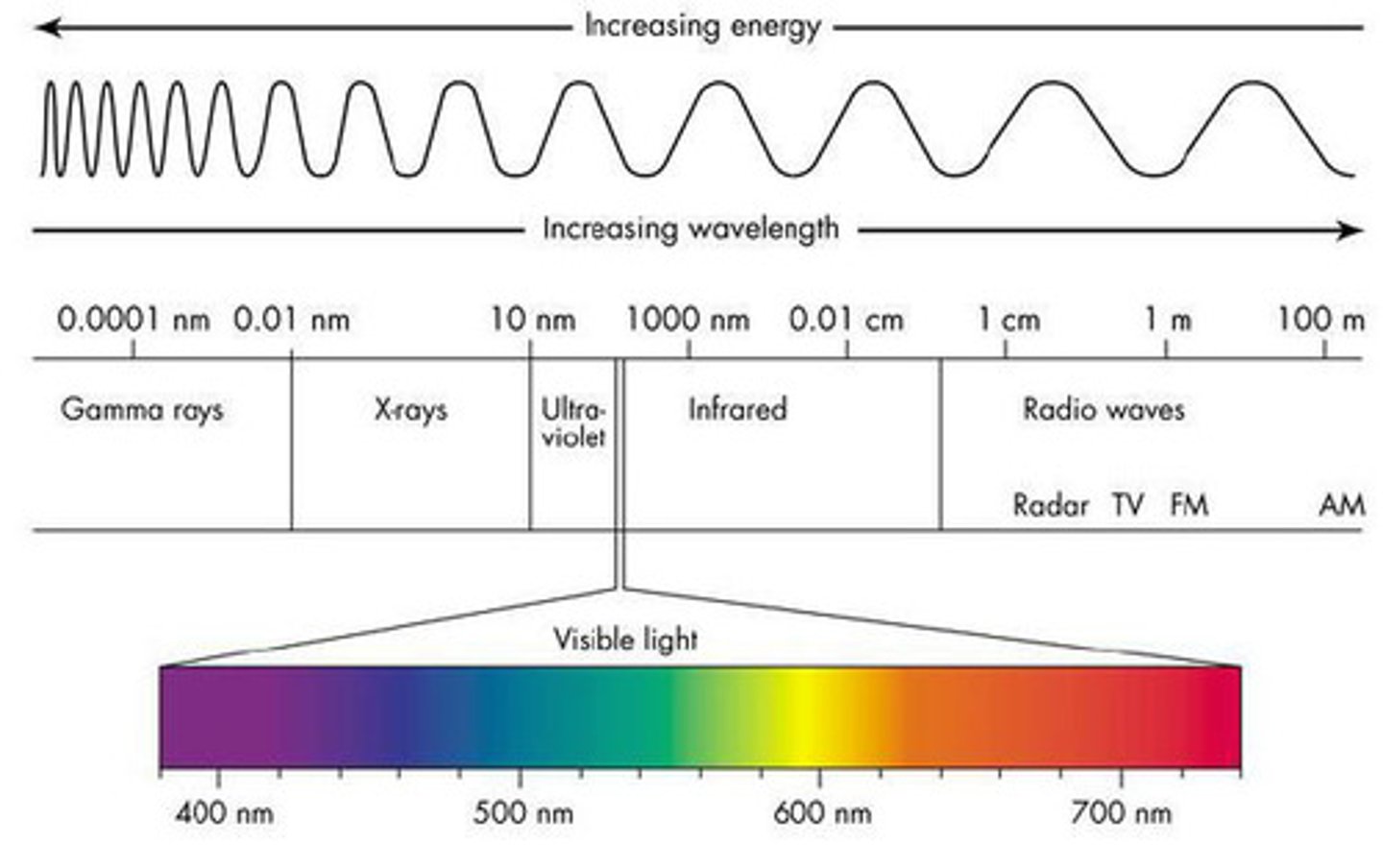
highest to lowest energy wavelength
gamma rays > X rays > UV > visible > IR > microwave > radio waves
Spectrophotometer
Measures absorbance or transmittance of light, as a function of wavelength.
monochromator
prism that splits white light from the lamp into its constituent wavelengths
TRUE OR FALSE
molecules don't absorb light
FALSE, particles or bonds in the molecule can jump to an excited state
transmittance (T)
amount of light making it through the sample (I0/It)
range of transmittance (T)
0 to 100%
eq absorbance
Aλ = log (I0/It)
what is I0
initial light
what is It
transmitted light
what does beer lambert law states
Absorbance is directly proportional to concentration
eq beer lambert law
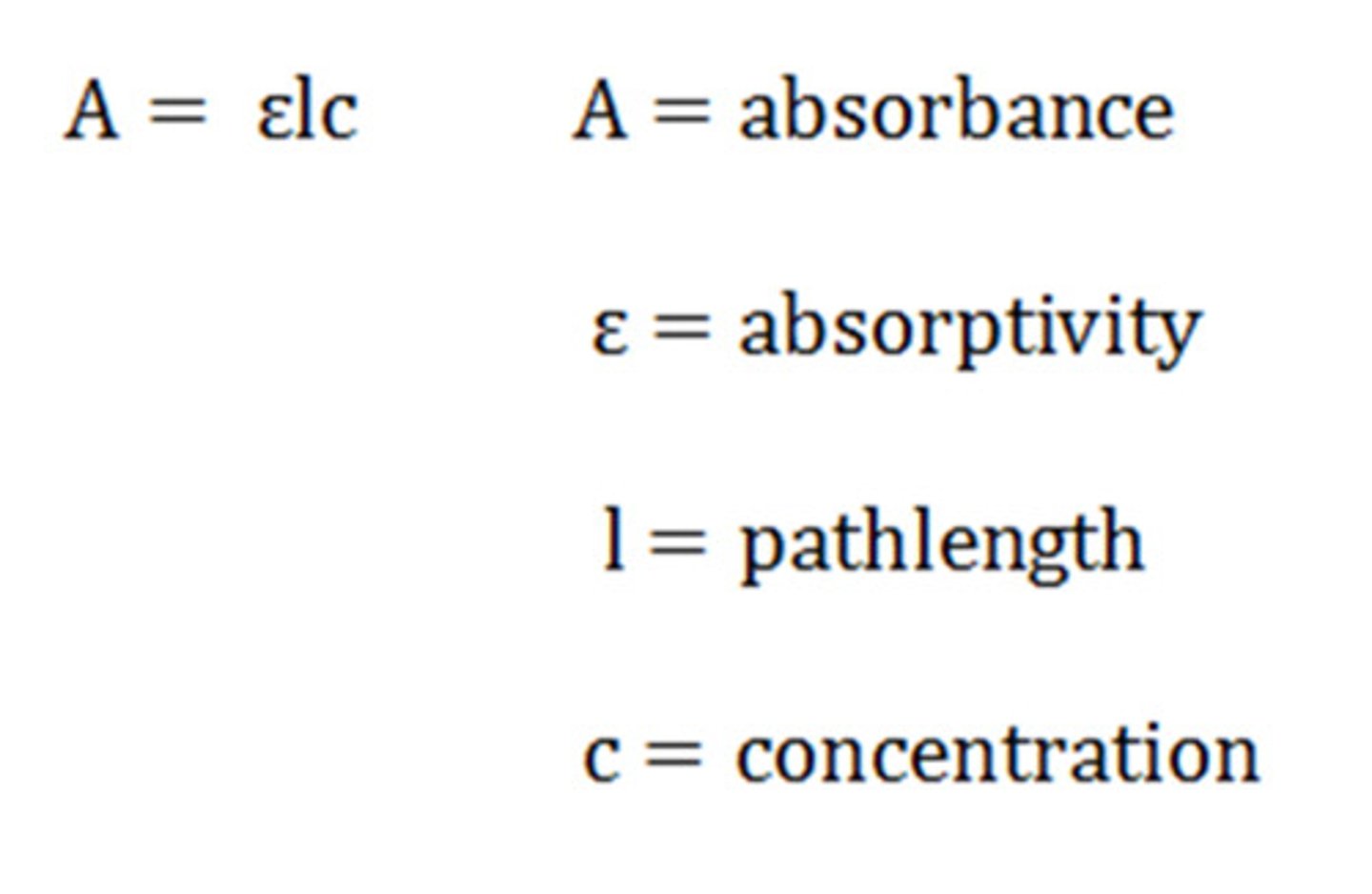
what is A in beer lambert law
absorbance
what is e in beer lambert law
molar absorptivity or extinction coefficient
TRUE OR FALSE
Absorbance has no units
TRUE
What is the unit of e in beer lambert law
Abs per unit conc. Per unit length (M-1 cm-1)
what is l in beer lambert law
path length of the sample, usually expressed in cm
What is c in beer lambert law?
concentration expressed in mol/L
what happens to the absorbance if the concentration is doubled
the abs is doubled
what is the negative control in the spectrophotometer
reference blank
what is the reference blank
Contains everything found in the sample solution except the substance being analyzed
Calibrates the absorbance reading A=0.000
what is the wavelength of Tyr, Phe and Trp
280 nm
wavelength of peptide bonds
200 nm
what is Biuret Reagent made of
Alkaline solution of Copper sulfate (CuSO4)
how does biuret reagent work
Reacts with NH of peptide bond
less sensitive
Good for solutions with high protein concentration
TRUE OR FALSE
Biuret reagent is not concentration dependent
FALSE , Biuret reagent reacts with peptide bonds, and its colour intensity is directly proportional to the protein concentration.
what happens when copper reacts with the protein in biuret reagent
colour change from blue to violet
what is the wavelength for BSA (Bovine Serum Albumin
540 nm
the absorbance of an NADH solution at 340 nm is 0.19 AU. The extinction coefficient (e) of NADH is 6300 M-1 cm-1. What is the NADH concentration in µM? (path length = 1 cm)
30.2 µM
C = A / (e * l), where C is concentration, A is absorbance, e is the extinction coefficient, and l is the path length.
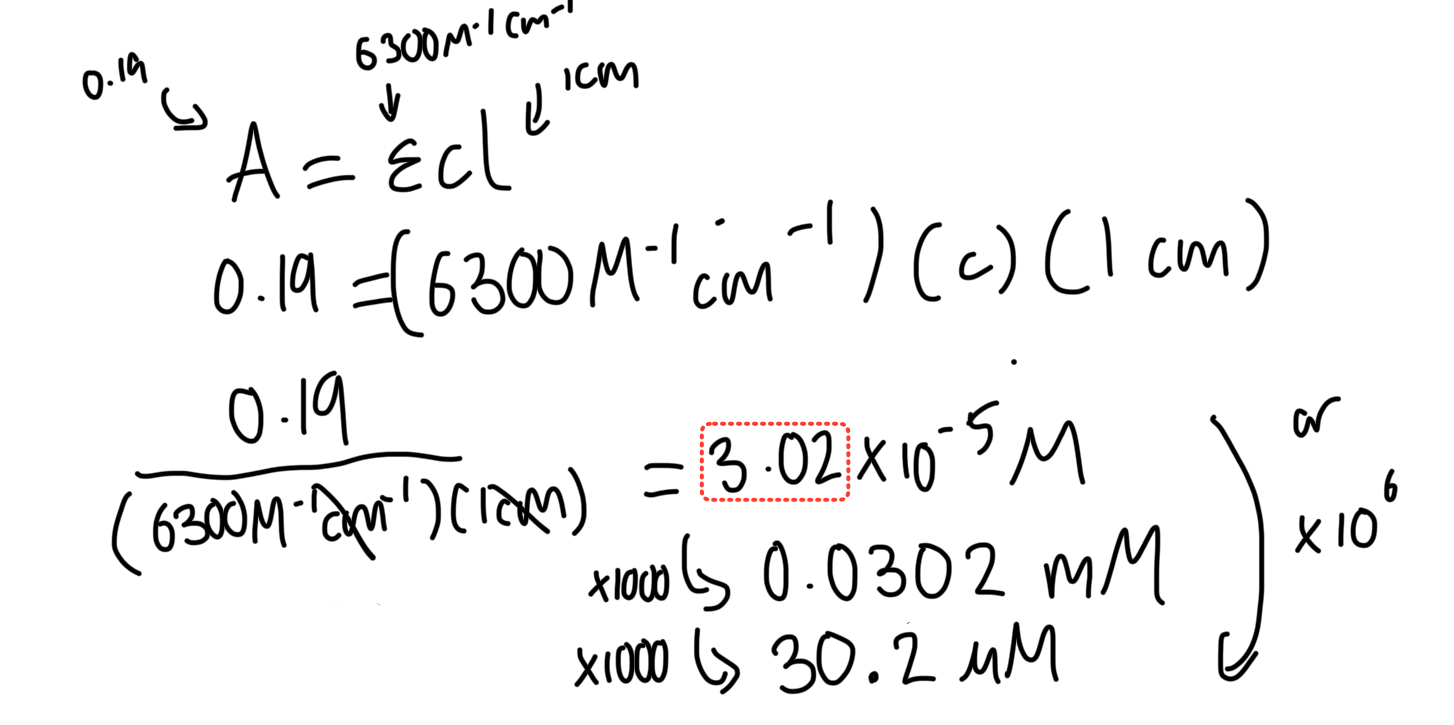
you have 5% w/v stock solution of BSA you want to make 5 mL of a 3.5% solution. How much of the stock solution do you need?
3.5 mL
What is the name of the reagent used to determine protein concentration?
biuret reagent
f = c/lambda, name each term in this equation
f = frequency
c = speed of light
lambda = wavelength
which of the following correctly orders the EM radiation types from lowest to highest energy?
A) microwave, radio waves, X rays, UV
B) y-rays, radio waves, IR, UV
C) radio waves, visible, UV, IR
D) microwave, IR, visible, UV
D) microwave, IR, visible, UV
relationship between wavelength and frequency
the higher the energy, the lower the wavelength
what pH range does biuret reagent work best
Biuret reagent only works best on alkali solutions