rxn rules
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
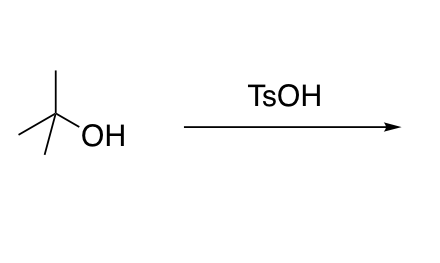
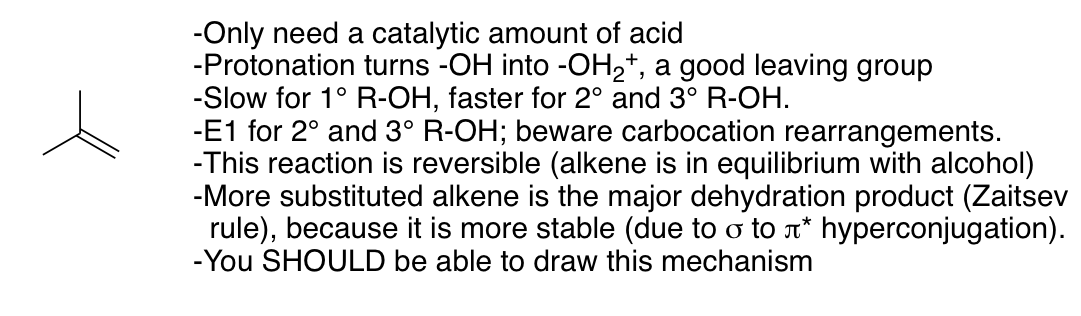
Dehydration of alcohols under strongly acidic conditions
Sn1 only with carbocation intermediate
H-Cl, H-Br, H-I as nucleophile/bases attacking an alcohol
Step 1: protonation of alcohol, generating our nucleophile as well
Step 2: leaving group leaves, generation of carbocation
Step 3: Consider any possible carbocation rearrangement
Step 4: Substitution (Sn1) via attacking LUMO p-orbitals with Br, Cl, or I minus
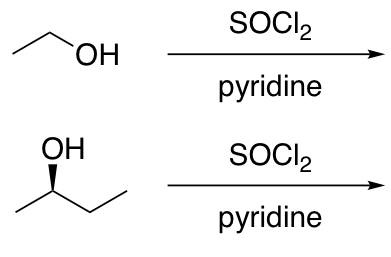
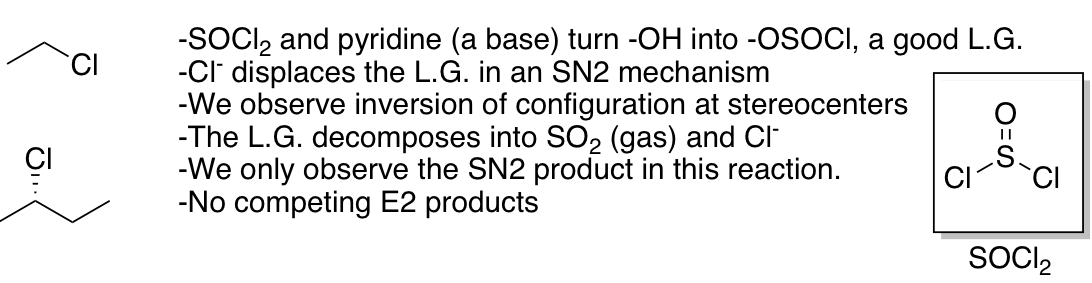
Substitution of 1° and 2° alcohols with halides under conditions that do not allow carbocation rearrangement
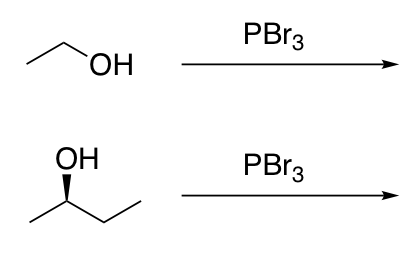

Substitution of 1° and 2° alcohols with halides under conditions that do not allow carbocation rearrangement
Sn2
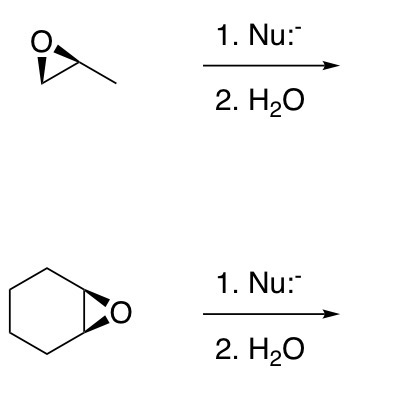

Opening of epoxides with strong nucleophiles (-OH, -OR, -CN, -N3, -SR, NH3)
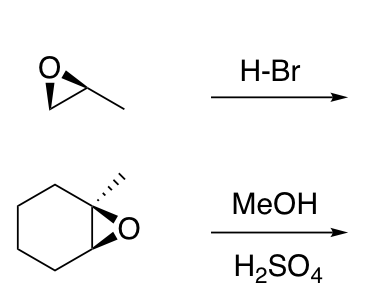
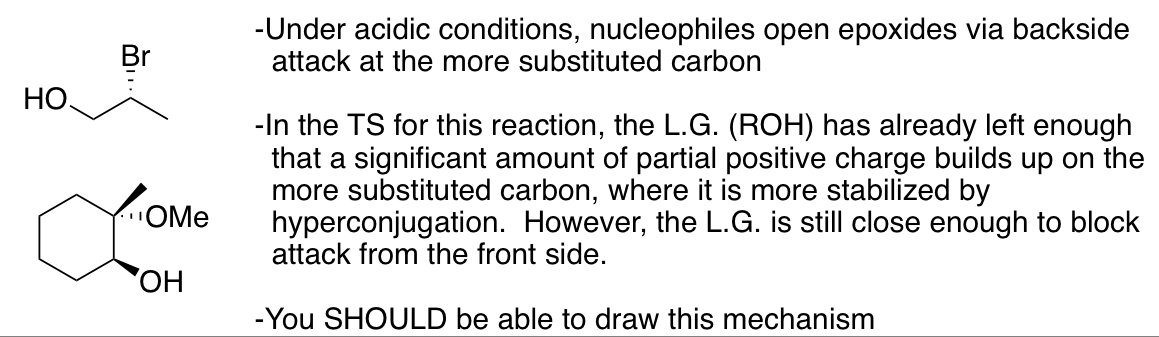
Opening of epoxides with H-Z (Z = Cl, Br, I, H2O, ROH)
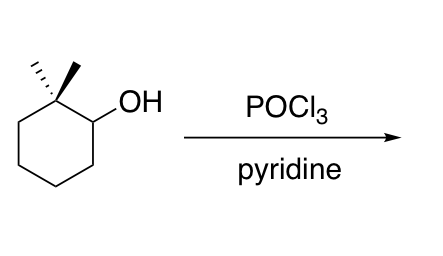

Dehydration of alcohols with POCl3 / pyridine
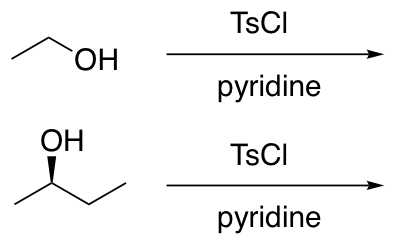
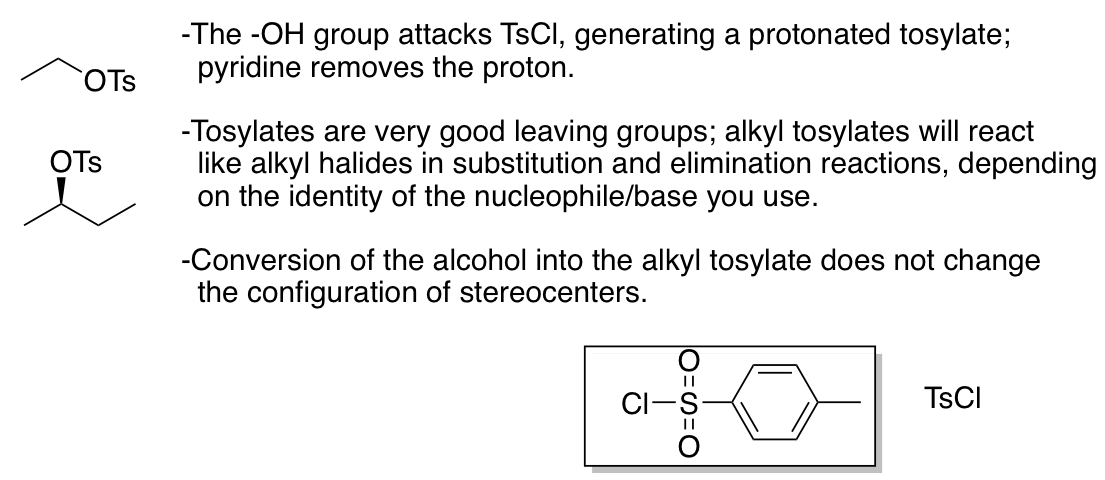
Conversion of alcohols into alkyl tosylates (tosylate is a good L.G.; more or less equivalent to a halide)
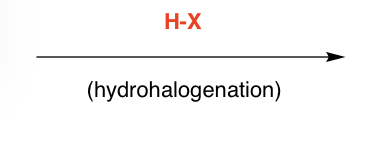

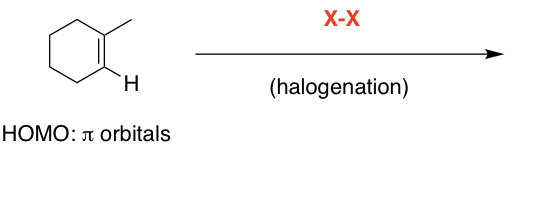
H-X
H on less substituted, +on more substituted, followed by attack by halide from both sides
Syn and anti

syn + anti
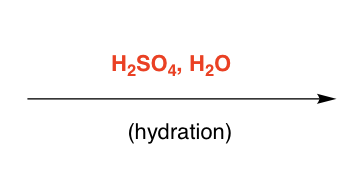
H2SO4, H2O
H on less subbed, +on more subbed
H2O attacks CI, deprotonated
Stereospecificity: none, attack either side
Syn and anti


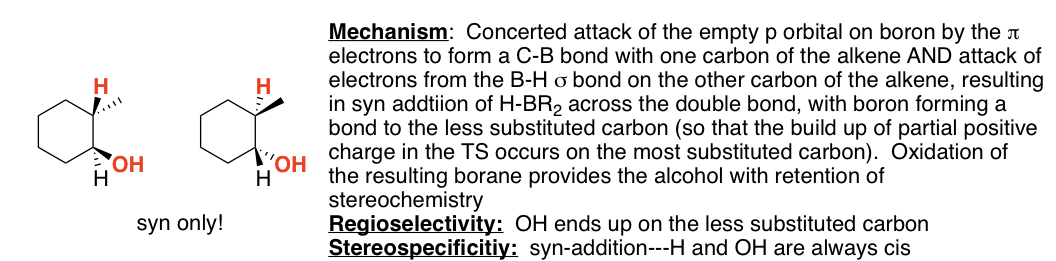
SN2 only
PBr3, SOCl2,
Epoxide w/ strong nucleophile
E1 only with carbocation intermediate
TsOH, H2SO4
Step 1: protonation of alcohol
Step 2: leaving group leaves, generation of carbocation
Step 3: Consider any possible carbocation rearrangement
Step 4: Elimination (E1) via attacking beta protons
E2 only, no carbocationic intermediate
POCl3