Basic Chemistry: Specific Heat Calculations and Applications
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What is specific heat?
The amount of heat needed to change the temperature of 1 gram of a substance by 1 °Celsius.
What are the units of specific heat in the SI system?
Joule/gram °Celsius.
What are the units of specific heat in the metric system?
calorie/gram °Celsius.
What is the formula to calculate specific heat (S H)?
S H = q / (m * ΔT), where q is heat in joules, m is mass in grams, and ΔT is the temperature change.

How does the specific heat of water compare to that of rock?
Water can absorb or release five times the energy of the same amount of rock, moderating temperatures.
What is the specific heat of lead if 57.0 Joules raises the temperature of 35.6 grams by 12.5 °C?
Specific heat of lead = 0.127 Joule/gram °Celsius.
What is the heat equation derived from specific heat?
q = m S H ΔT, where q is the heat lost or gained.
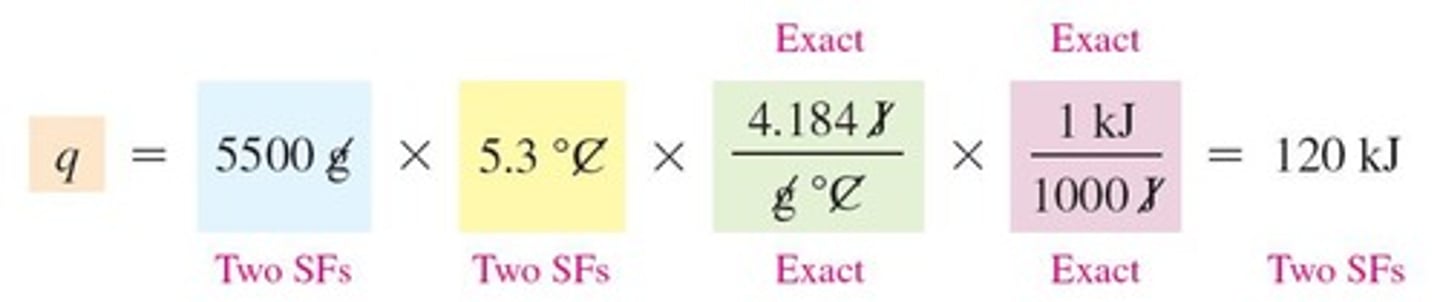
What is ΔT in the context of specific heat?
ΔT is the temperature change calculated as final temperature minus initial temperature.
In a cooling cap scenario, why is body temperature lowered?
To reduce the amount of oxygen needed by the body during surgery or after a cardiac event.
How do you calculate the specific heat of chromium when mixed with water?
Use the heat exchange principle where all heat lost by chromium equals heat gained by water.
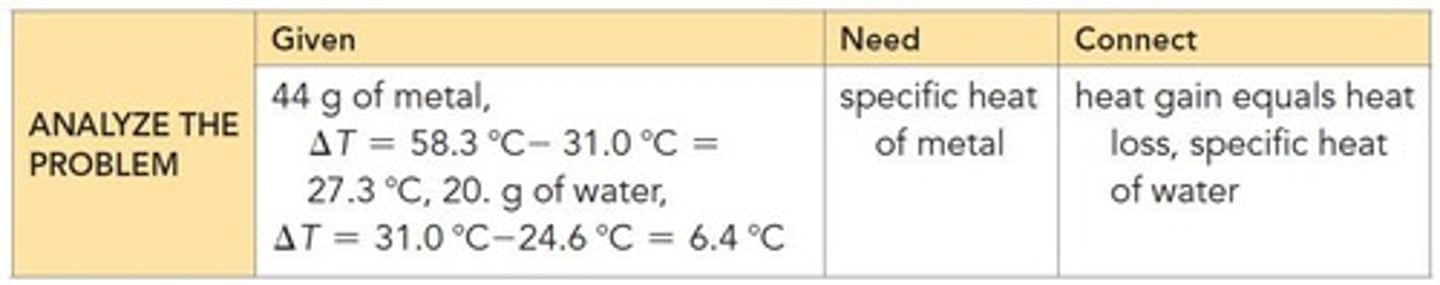
What is the specific heat review goal?
To calculate the specific heat for a substance and use it to calculate heat loss or gain.
What is the relationship between mass, temperature change, and specific heat?
The heat lost or gained by a substance is determined by multiplying its mass, the temperature change, and its specific heat.
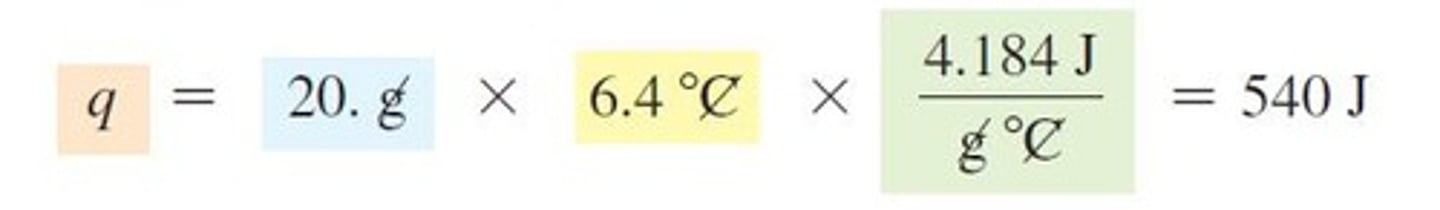
What happens to the temperature of water in coastal areas compared to inland areas?
The high specific heat of water keeps temperatures more moderate in coastal areas.
What is the significance of specific heat in everyday applications?
It helps in understanding how substances absorb and release heat, impacting temperature regulation.
What is the specific heat of water?
The specific heat of water is approximately 4.18 Joule/gram °Celsius.
Why is specific heat important in chemistry?
It is crucial for calculations involving heat transfer in chemical reactions and physical processes.
What does a high specific heat indicate about a substance?
It indicates that the substance can absorb a lot of heat without a significant change in temperature.
What is the effect of specific heat on climate?
Substances with high specific heat can moderate climate by absorbing heat during the day and releasing it at night.
What is the specific heat of metals generally compared to water?
Metals typically have lower specific heats than water, meaning they heat up and cool down more quickly.