Haematopoietic Stem Cell Transplantation and Graft vs Host Disease
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
HSCs can be obtained in three main ways. Which one is most commonly used?
Bone marrow (previously used)
Peripheral blood
Umbilical cord
HSCs are used in the treatment of which two types of diseases? What is the aim of HSC transplantation in these cases?
Haematological malignancies (e.g. leukaemia, multiple myeloma)- to replace abnormal immune cells
Immune-deficiencies (SCID, WAS)- to replace lack of cells
What is the main clinical complication following HSC transplantation?
GvHD
Allogeneic HSCs can cause the ______ effect which can be used to treat haematological malignancies such as leukaemia. However, this effect may be negative as it can cause ______.
GvL (graft vs leukaemia)
Donor stem cell preparation contains alloreactive T cells that recognise alloantigens on tumour cells.
BUT can also attack alloantigens in recipient tissues (GvHD)
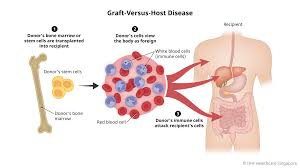
What is GvHD?
Graft-versus-host disease (GVHD) is a severe complication that can occur following hematopoietic stem cell transplantation.
Immunocompetent allogeneic T cells from the donor graft recognize the recipient's tissues as foreign due to histocompatibility differences and initiate an immune response against them.
How can GvHD be prevented and what is the side effect?
T cell depletion of HSCs via irradiation to remove alloreactive T cells
BUT this causes loss of GvL effect
Transfusion-associated GvHD is rare, but also possible. How can this be prevented?
screening blood products for functional alloreactive T cells
What causes GvHD?
Mismatches in minor or major (usually minor- MiHAs) histocompatibility antigens.
GvHD requires three specific conditions:
Graft contains immunocompetent (functional) alloreactive T cells
Mismatch of major/and or minor histocompatibility antigens
Recipient is incapable of rejecting the graft (have no immune cells due to chemo/radiotherapy)
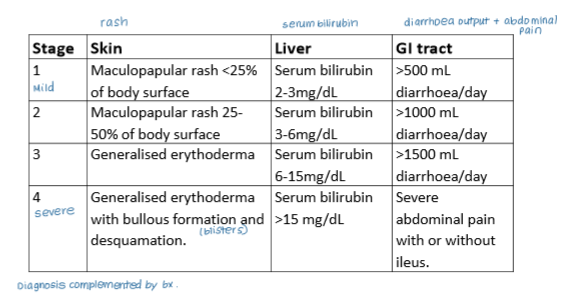
What are the three main organs affected by GvHD and how are changes in these organs reflected in clinical staging of the disease?
Skin (rash)
GI tract (diarrhoea)
Liver (serum bilirubin)
What is the difference between acute and chronic GvHD and how are they differentiated?
Old definition (days post-transplant):
Acute- symptoms appear <100 days post-transplant
Chronic- >100 days post-transplant
New definition (histological features):
Acute- epithelial cell death
Chronic- fibrosis and atrophy
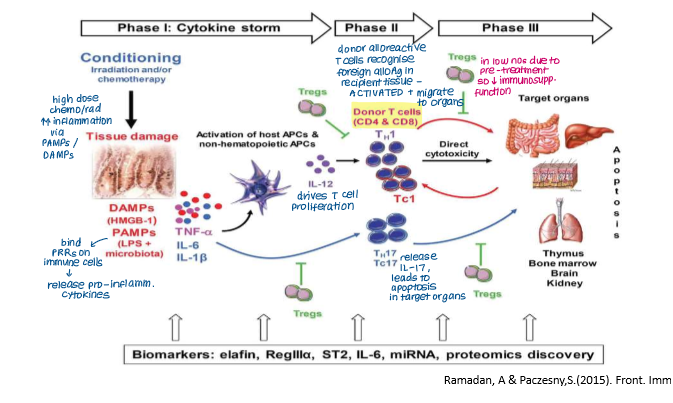
Acute GvHD has three main phases:
Conditioning (creating inflammatory environment pre-transplant)
Priming and activation of alloreactive donor T cells
Effector phase
Explain what happens in the first phase of acute GvHD
Conditioning (Tissue Damage)
Chemotherapy/radiotherapy damages recipient tissue (skin, GI tract, liver)
Pro-inflammatory cytokines (TNF-a, IL-6, IL-1) released by patient cells in response to DAMPs and PAMPs (PAMPs from gut microflora)
Cytokines activate patient APCs which present alloantigens to alloreactive T cells in graft, priming and activating them.
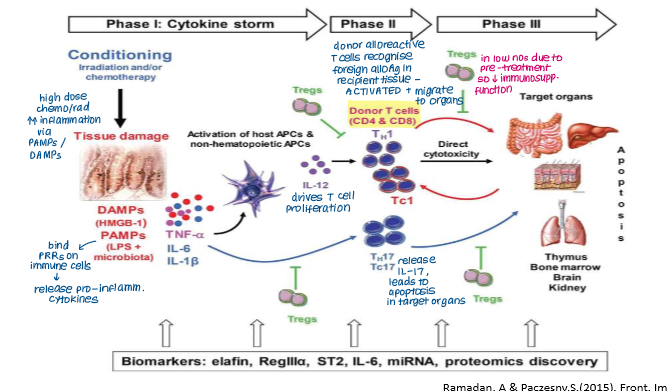
Explain what happens in the second phase of acute GvHD
Priming and Activation of Alloreactive T Cells
Alloreactive T cells in graft are primed and activated by patient APCs presenting alloantigens
Thymus- alloreactive T cells damage mTECs, disrupts tolerance
GI, skin, liver- patient’s APCs promote activation of alloreactive T cells
Differentiation
Cytokine release causes differentiation of alloreactive donor T cells into inflammatory cells (Th1/2, Tc, Th17 cells) that promote further cytokine release or apoptosis
Decline in Tregs so lose immunosuppressive function
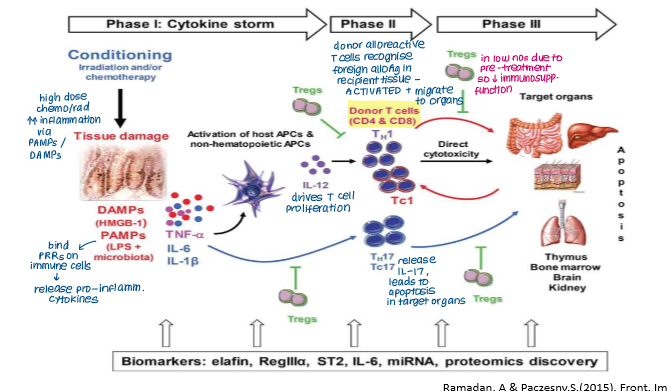
Th17 cells are important because?
Secrete IL-17 → APOPTOSIS
In the priming and activation phase of aGvHD, what are the two main sites of activity and which T cells are activated in each site?
Thymus: activation of CD4+ T cells via haematopoietic-derived APCs (e.g. dendritic cells, macrophages)
GI tract: activation of CD8+ T cells via non-haematopoietic APCs (e.g. epithelial cells)
Explain what happens in the final stage of acute GvHD
Effector phase
Differentiated alloreactive donor T cells (e.g. Th1/2, Tc, Th17) are recruited into patient tissues by chemokines released by tissue APCs
Causes tissue damage by:
Cytokines TNF-a and IL-1 from activated APCs mediate apoptosis
CTLs directly destroy patient cells expressing self-HLA molecules
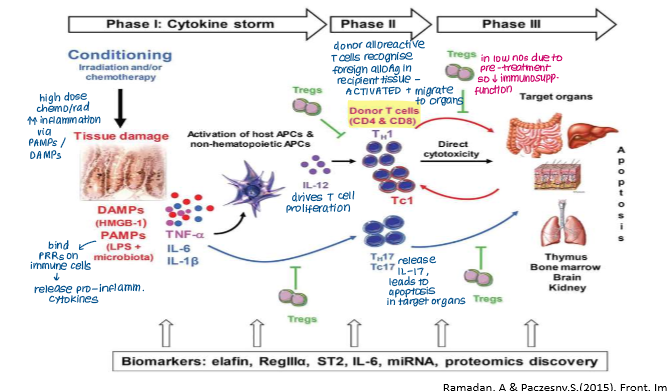
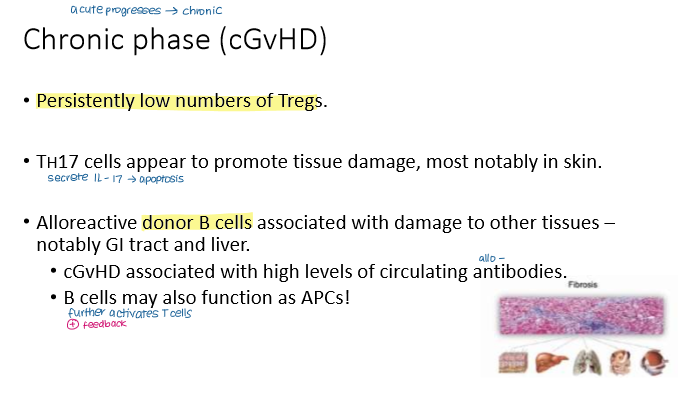
Which of these is not a common feature of chronic GvHD?
a) Low numbers of Treg cells
b) Increased numbers of Th17 cells
c) Increased numbers of CD8⁺ mediated epithelial apoptosis in the GI tract
d) Alloreactive donor B cells secrete alloantibodies
c) Increased numbers of CD8⁺ mediated epithelial apoptosis in the GI tract
How can GvHD be prevented?
Donor selection (high resolution HLA typing of major and minor HA, sex-matching, alloimmunisation to MiHA from foetus)
CMV screening
Irradiation to deplete alloreactive T cells from graft (BUT lose GvL effect)
Use of autologous HSCs where possible
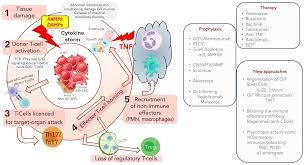
How can GvHD be treated?
Anti-rejection drugs (corticosteroids, classical anti-rejection drugs)
Monoclonal antibodies
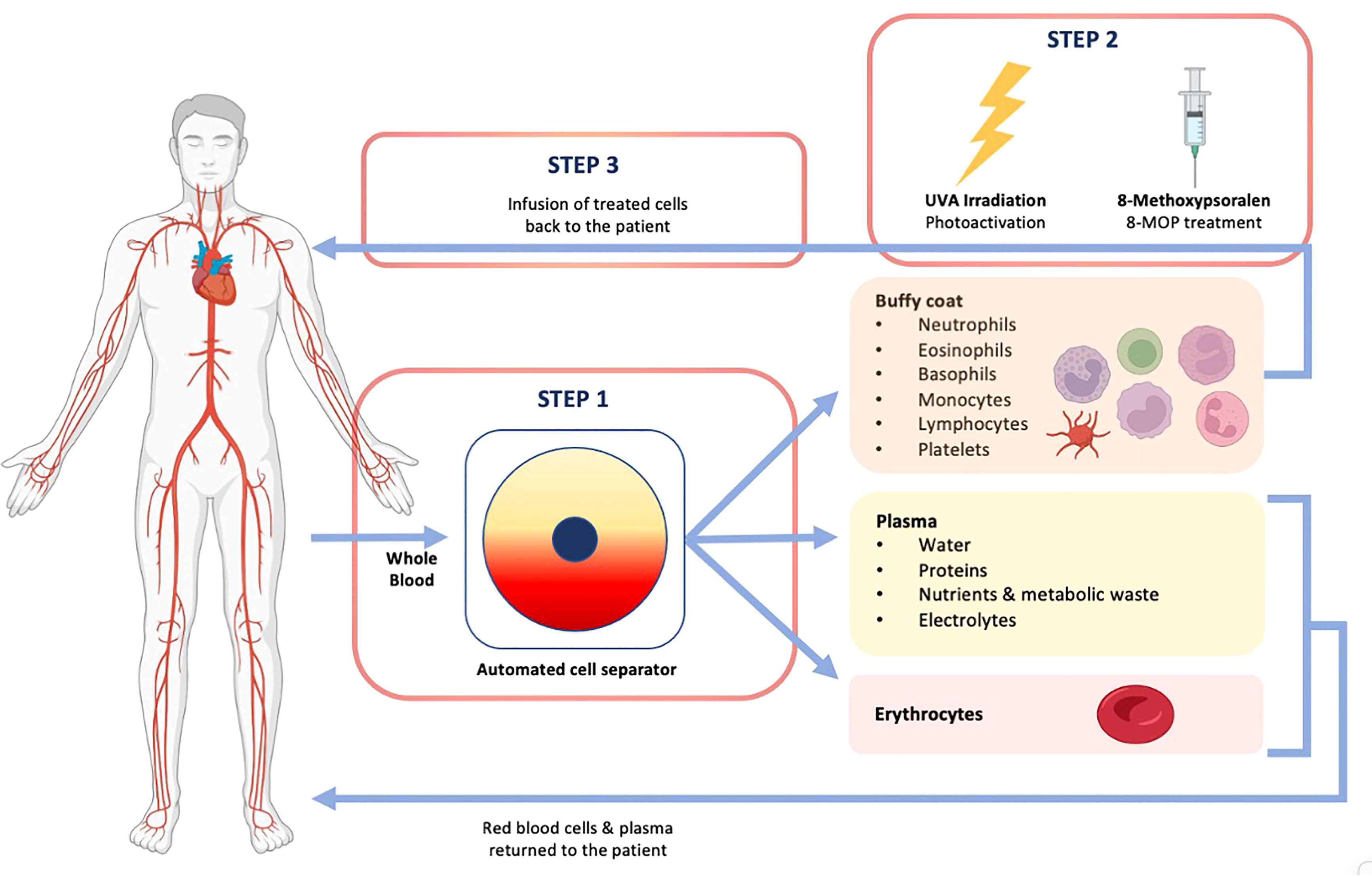
Extracorporeal photophoresis (EP)- induces tolerance without immunosuppression (UV damages DNA in lymphocytes, causes apoptosis)