Intro to Occlusion Exam 1 [Alginate and Gypsum]
1/81
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
82 Terms
Occlusion
The static relationship between the incising or masticating surfaces of the maxillary or mandibular teeth or tooth analogues
Articulation
The static and dynamic contact relationship between the occlusal surfaces of the teeth during function
Alginate is an
irreversible hydrocolloid
Alginate is a commonly used material for the ________ of hard and soft tissue
registration (impression)
Uses of Alginate:
Study casts
Preliminary casts for making custom trays
Definitive removable partial denture impressions
Alginate: SOL-GEL reaction
Sol: Suspension of dispersion of larger particle in a solvent
Gel: A colloidal system in which solid and liquid phases are continuous
Alginic acid
Mucous derived from seaweed is rich in alginic acid, which is a copolymer of
- anhydro-B-D-Mannuronic acid
- anhydro-B-L-Guluronic acid
Insoluble in water except when it is in its salt form (chelated with Na or K)
Alginic acid must be in ___ form to dissolve in water
salt form (chelated with Na, K)
When the salt form of Alginic acid mixes with water, a
viscous sol is formed which acts to create a supporting matrix or scaffold
The chelated for of Alginic acid =
Potassium alginate
Setting reaction:
Calcium sulfate + Potassium alginate = Insoluble calcium alginate
Potassium alginate
Alginic acid in salt form so that it is soluble in water to for a sol
Function:
- Soluble alginate
Weight percentage
- 15
Calcium sulfate
Calcium sulfate is the reactor:
- Provides calcium ions to the alginate scaffold
Function:
- Reactor
Weight percentage
- 16
Filler particles
Diatomaceous earth and Zinc oxide are your fillers
Diatomaceous earth
Acts as a filler
- Increases strength and stiffness
- Smoother texture
- Firm surface
Function:
- Filler particles
Weight percent:
- 60
zinc oxide
Also a filler
Function:
- Filler particles
Weight percent
- 4
Potassium titanium fluoride
Helps accelerate the setting of stone once poured
- Creates a hard, dense surface
Function:
- Gypsum hardener
Weight percent:
- 3
Sodium phoshate
The potassium ions in the alginate scaffold are replaced with calcium very rapidly
Sodium phosphate acts as a retarder and defers this reaction, so that the working time is sufficient
Function:
- Retarder
Weight percent
- 2
Setting reaction: described
Potassium alginate with water forms a viscous Sol scaffold
Calcium ions replace the potassium ions to cross link and stabilize the scaffold
The production of insoluble calcium alginate is too rapid for practical use- so we must
add a retarder to slow this down and give us more working time
We use sodium phosphate:
- Phosphate ions preferentially consumes calcium
Elastic recovery
How well an impression material is able to maintain its original shape after it is taken out of the patients mouth
Alginate has _____ elastic recovery
poor
Other materials are better suited for elastic recovery
Alginate's flexibility is ____ compared to other impression materials
better
Reproduction limit
Have a groove that is 25 microns thick, and you impress that groove with your impression material. If you are able to accurately replicate that 25 micron groove then you have a good ability to replicate what you need.
Alginates Reproduction limit compared to other impression materials is
Poor
Alginates tear strength compared to other impression materials
Poor
Advantages of Alginate
Non toxic
Accurate
Inexpensive
Easy to manipulate
Disadvantages of Alginate
Non-correctable
Give clinically overextended borders
Compresses soft tissue
Must pour immediately
Can react (etch) the gypsum cast
EFFECT: Grainy material
Improper mixing
Excessive gelation
Water/Powder ratio too low
EFFECT: Tearing
Premature removal from the mouth
Moisture contamination
Prolonged mixing
EFFECT: Bubbles
Air incorporation during mixing
Moisture or debris on tissue
EFFECT: Distortion
Impression not poured immediately
Moving the tray during gelation
Premature/improper removal from the mouth
ADA Standards
Not on study guide
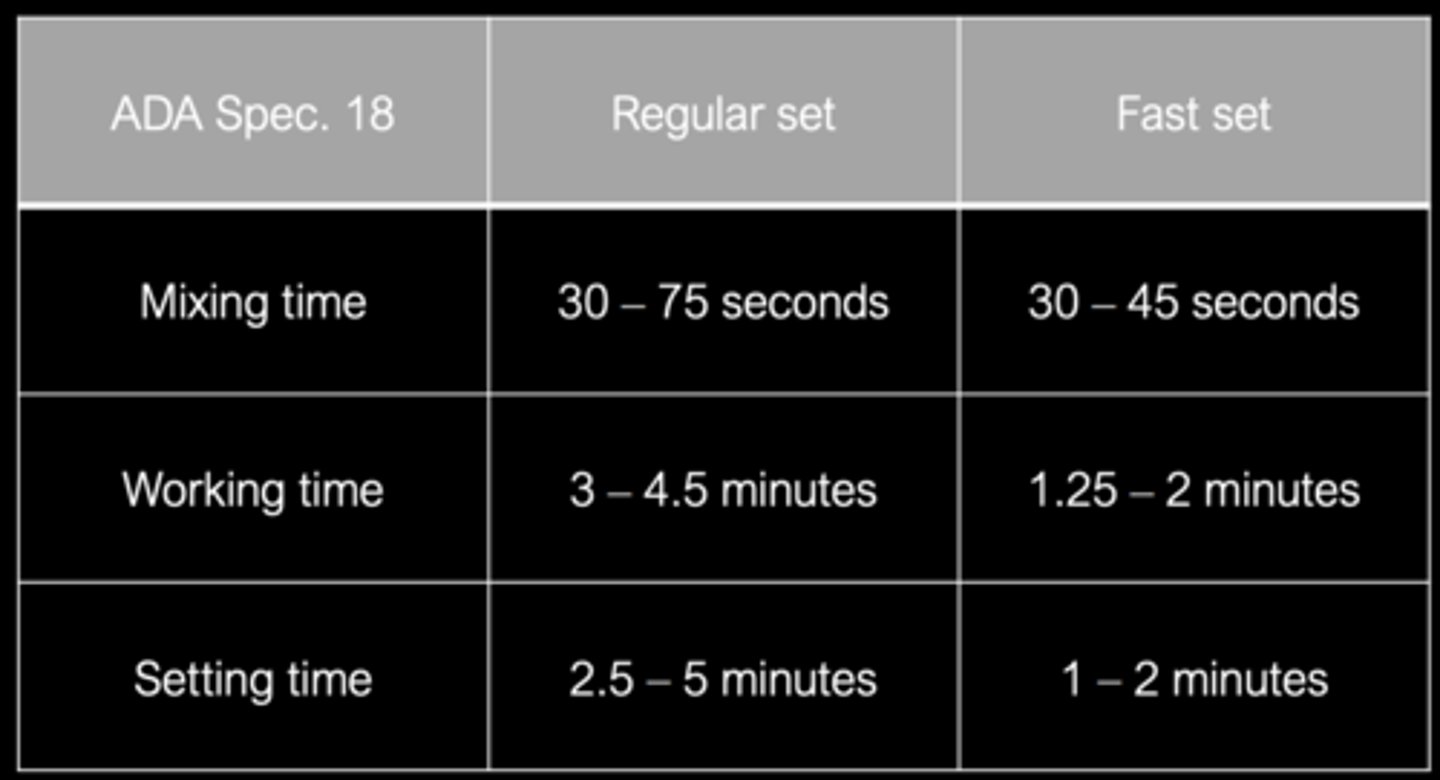
Alginate impressions should be poured within ___ to avoid syneresis and imbibition
12 minutes
Syneresis
Calcium organizes the water in the "sol" scaffold as it turns to "gel"
Squeezes water out
Continue to lose water after alginate sets
Limit its effect on dimensional stability by pouring impression immediately after removal from mouth
TIP:
Syneresis = Shriveled (both start with S)
Imbibition
Gel state can absorb water
Also causes dimensional change
Similar to osmosis
TIP:
ImBIBition
(wear a BIB when you eat which makes you bigger)
Handling rules
Use distilled water
Use room temperature water
Mix proper amount of time
Remove with a snap
Store in a sealed plastic bag
Pour within 12 minutes
WHY THE RULES: Distilled water
Hardness range
WHY THE RULES: Room temp water
Setting time changes
WHY THE RULES: Mix proper amount of time
Otherwise grainy
WHY THE RULES: Remove with snap
Elastic recovery, compressive and tear strength all improved
WHY THE RULES: Store in sealed plastic bag
100% relative humidity
WHY THE RULES: pour within 12 minutes
Avoid syneresis
Changing water:powder ratio
Changes consistency
Strength (thicker = stronger)
Working and setting time (thicker = faster)
Flow (thicker= more voids and less detail for teeth)
Soft tissue displacement (thicker = pushes lips and cheeks more)
Changing water: powder ration
More thick means
more powder
Gypsum uses
Impression plasters
Model stones
Die materials
Binder for silica investments
Gypsum is composed of
calcium sulfate and water
Gypsum can exist in
dihydrate and hemihydrate forms
Gympsum is typically mined as the
dihydrate
Calcination
Heating process performed by the manufacturer to create a hemihydrate powder form of the product
This hemihydrate form is what we use in dentistry
The _____ form is what we use in dentistry
Hemihydrate
Type I
Impression plaster
Type II
Model plaster
Type III
Dental stone
Type IV
High strength stone
Type V
High strength and high expansion stone
All type of Gypsum classification
have the same chemical formation
Physical properties of Gypsum classifications are derived from how the
water is driven off in the calcination reaction
What type of gypsum classification do we use in the lab to pour our cast
Type III
Calcination: reaction
CaSO4 , 2H2O (Mined dihydrate) --> CaSO4 , 1/2 H2O (Hemihydrate powder)
Hemihydrate can be classified based on the physical form of the actual powder particles
Beta-hemihydrate
Alpha- hemihydrate
Modified alpha-hemihydrate
Beta-hemihydrate
Plaster
Rough and irregular particle shapes

Alpha-hemihydrate
Stone
Uniform, dense particle

Modified alpha-hemihydrate
Die stone
Shorter and thicker particles
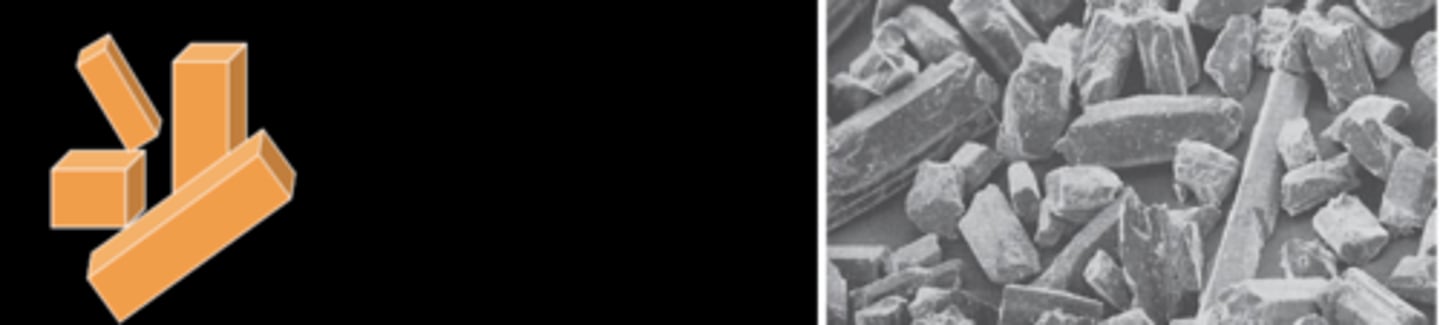
Type I: Impression plaster
Type II: Model Plaster
Beta-Hemihydrate
Type III: Dental Stone
Alpha-Hemihydrate
Type IV: High-strength stone
Type V: High-strength, high expansion stone
Modified Alpha-Hemihydrate
Manufacturing a processes of dehydration: Type II Plasters
Open kettle heating around 110-120 degrees celcius
Results in predominantly irregular shaped particles (Beta-hemihydrate)
Manufacturing a processes of dehydration: Type III Dental stones
Dehydration under pressure in the presence of water vapor to control the rate of water loss
Creates a more uniform particle shape (alpha-hemihydrate)
Manufacturing a processes of dehydration: Type IV and V die stones
Dehydration in the presence of 30% CaCl2
Milling of the final product is then performed to create a very uniform sized particle
All gypsum products set by
the same reaction
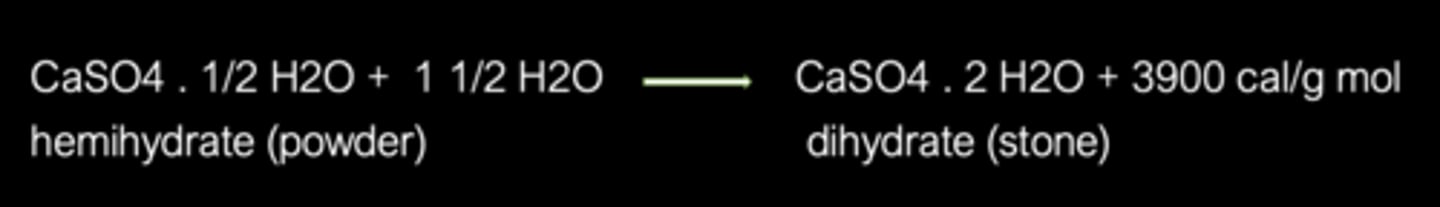
Chemically, 100g of hemihydrate requires only _____ mL water to form the dihydrate form
18.61
Derived primarily from the shape and form of the hemihydrate particles in the powder
The more uniform the particle size, the
less water is need to "Wet" the particles and thus the more dense, less porous the set mass

Setting expansion
All gypsum materials demonstrate some linear expansion upon setting

Compressive strength
Strength values increase from plasters to the die stones
Depends on the water/powder ration

Controlling setting time
Performed during manufacturing and clinically
Chemical modifiers like accelerators and retarders can be added by manufacturers to control setting time
Chemical modifiers: Accelerators
Potassium sulfate
Calcium sulfate dihydrate
Sodium chloride acts as an accelerator but also increases expansion
Chemical modifiers: Retarder
Borax
Controlling setting time Clinical modification
Adding slurry water (water saturated with fine gypsum particles) to accelerate setting
Changing the water/powder ratio- the high the ratio the more delayed the setting
Spatulation
water temperature
contamination
Spatulation
Mixing increases the number of nucleation sites, accelerating the setting reaction
Water temperature
Elevated temperature increases ion mobility which tends to offset the change in solubility
Contamination
Colloidal solutions block the expansion of the calcium sulfate dihydrate crystals leading to a soft porous surface to the stone
RINSE RINSE RINSE