Oxidation, reduction and Redox equations
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
15 Terms
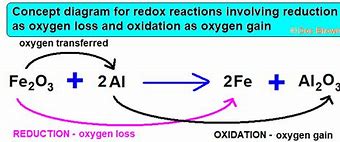
What is oxidation
The loss of electrons or the gain of oxygen
What is reduction
the gain of electrons or the loss of oxygen
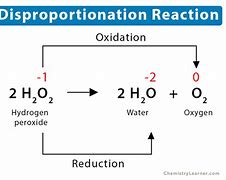
What is a disproportionation reaction
This is where a single substance is both oxidised and reduced
What is a redox reaction
This is where both oxidation and reduction occurs in a single reaction
What is the oxidation state of an element
The oxidation state is the number of electrons which must be added to a positive ion or the number of electrons which must be removed from a negative ion to give a neutral atom
What is the oxidation state for an uncombined element (Mg, H2,O2 etc)
0
What is the oxidation state for oxygen in general compounds and then in peroxides
General compounds ( MgO) O = -2
Peroxides (H2O2) O = -1
What is the oxidation state of hydrogen in compounds then in metal hydrides
In compounds (HCl) H = +1
In metal hydrides (NaH) H= -1
What is the oxidation states of group 1 metals and group 2 metals?
In group 1 metals (KCl) the metal in the compound (K) is always + 1
In group 2 metals (CaF) the metal in the compound (Ca) is always +2
What are the oxidation states of group 7 elements in compounds and in compounds where they are combined with a more electronegative element
In compounds (MgF2) F = -1
In compounds with a more electronegative element ( NaClO ) F = +1, Na = +1 O = -2
What are oxidising agents
These are substances which oxidise another substance and so are electron acceptors ( They themselves are reduced)
What are reducing agents?
These are substances which reduce another substance and so are electron donors ( they themselves are oxidised)
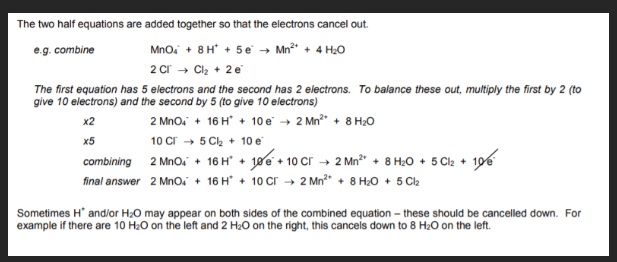
Steps for constructing half equations for redox reactions

example for constructing half equations for redox reactions

Combinding 2 half equations for an overall equation example