Galvanic cells
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
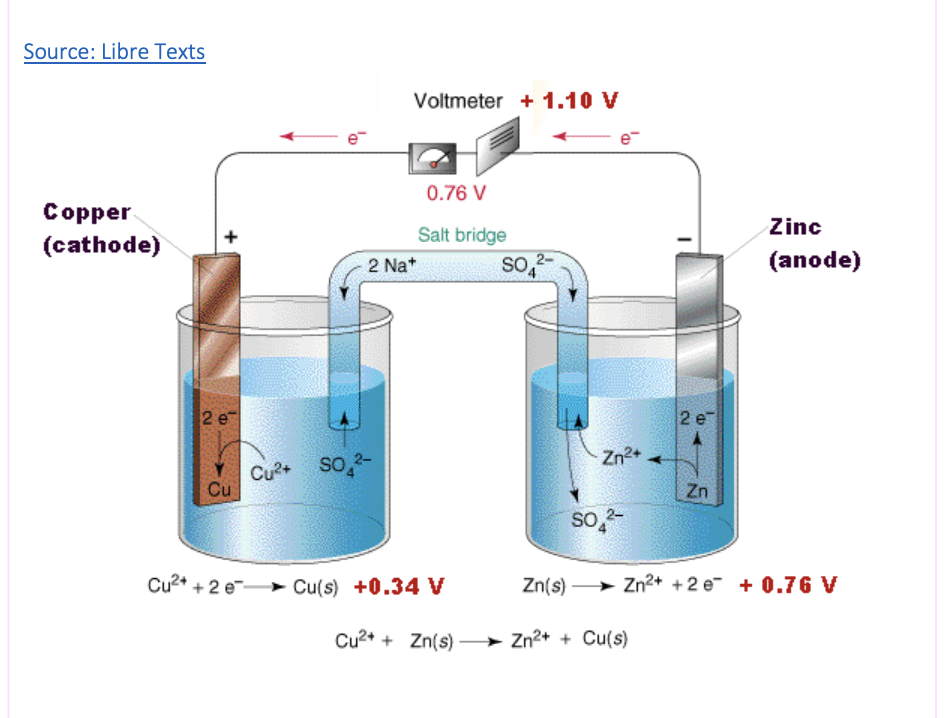
Components of galvanic cell: half-cells
where either oxidation or reduction occurs
e.g a beaker containing an ionic solution and an electrode. In the diagram on the right, the left half-cell contains copper sulphate solution with a copper electrode
Components of a galvanic cell: anode and cathode
anode: the electrode at which oxidation occurs; the negative electrode (An ox)
cathode: the electrode at which reduction occurs; the positive electrode (Red cat)
Components of galvanic cell: external circuit
wires that create the circuit and allows for electrons to move from the anode to the cathode *can include a voltmeter in the external circuit
Components of galvanic cell: salt bridge - includes the movement of ions
connects the 2 half-cells and the movement of ions to balance the charge due to the redox reactions that are occuring.
e.g negative ions in the salt bridge move to the right to balance the positive charge formed in the half cell when the zinc is oxidised to Zn2+
How do fuel cells an applicant of galvanic cells and how they are used?
fuel cells use hydrogen and oxygen to produce water
electrons travel through a wire, providing
hydrogen fuel cells can operate under acidic or alkaline conditions
What is the purpose of galvanic cells?
produces electricity by a spontaneous redox reaction
2 half reactions = connected by a salt bridge and external wires
oxidation occurs in one half cell and reduction in the other