1.6 - Thermodynamics
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Define the Enthalpy of Formation
The enthalpy change when 1 mole of a compound is formed from its elements in their standard states under standard conditions
Define the Enthalpy of Atomisation of:
Element
Compound
Element:
Enthalpy change when 1 mole of gaseous atoms is formed from an element in its standard state
Compound:
Enthalpy change when 1 mole of compound in its standard state is converted into gaseous atoms
Define the Enthalpy of Ionisation
The enthalpy change when 1 mole of (higher charged) positvely charged gaseous ions are formed from 1 mole of gaseous atoms/ lower charged ions
Define the Electron Affinity
The enthalpy change when 1 mole of negatively charged gaseous ions are formed from 1 mole of gaseous atoms/lower charged ions
Define the Enthalpy change of Hydration:
Is it Endo or Exo (+why)
2 factors (how)
The enthalpy change when 1 mole of aqueous ions is formed from 1 mole of gaseous ions:
Always Exothermic as ion-dipole forces are being made (bond making = exo)
2 Factors:
Ion size = Larger = Lower charge density = Lower enthalpy of Hydration
Charge of ions = Larger = Higher charge density = Higher enthalpy of Hydration
Define the Enthalpy change of Solution
The enthalpy change when 1 mole of solute (ionic solid) is dissolved in enough solvent (water) such that no further enthalpy change occurs despite further dilution
Define the Enthalpy of Lattice Dissociation (exo or endo)
Enthalpy change when 1 mole of a solid ionic lattice is broken into 1 mole of its gaseous ions:
ENDO
Define the Enthalpy of Lattice Formation (exo or endo)
Enthalpy change when 1 mole of a solid ionic lattice is formed from 1 mole of its gaseous ions:
EXO
Why is the 1st electron affinity different to further affinities?
Include exo + endo in definition
Adding an electron is exothermic as attraction from the atoms nucleus is larger than electron repulsion - therefore since energy needed to remove electrons, energy is released to add an electron
EXO
Other affinities are adding additional electrons to -ve ions the repulsion>attraction energy is now needed to “absorb” the electrons
ENDO
What are Hydrated ions?
Ions formed when salt is dissolved in water and bond to polar water molecules
What 2 enthalpies determine a salts -ve/+ve enthalpy of solution? (how)
Enthalpy of lattice dissociation (endo) + Bond making of Ion dipole forces aka. Hydrated ions (exo):
The sum of these 2 determine the salts overall sign and its enthalpy nature (endo or exo)
BORN HABER CYCLES USE CHEM NOTES 2
BORN HABER CYCLES USE CHEM NOTES 2
What is Entropy?
Measure of disorder in a system, the number of ways particles and their energy can be arranged
Rank the states of matter by entropy (LEAST → MOST)
Solid
Liquid
Gas
Give the formula for entropy change
ΔS.system = ΣS.products - ΣS.reactants
Give the Gibbs Free energy change formula
ΔG = ΔH - TΔS
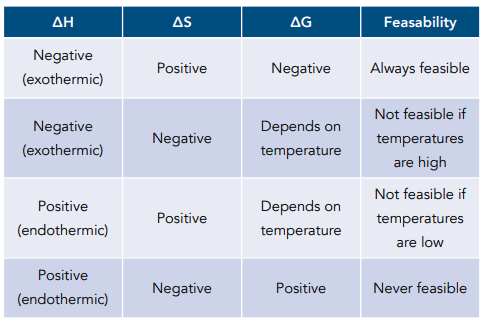
How can you determine if a reaction is feasible (ΔG)
ΔG < 0 (Negative): The reaction is feasible and likely to occur
ΔG > 0 (Positive): The reaction is not feasible
ΔG = 0: The reaction is at equilibrium, meaning it is just becoming feasible
How do you determine the minimum temperature when a reaction is feasible or not?
Set ΔG=0, then rearrange for T
List out the enthalpy, entropy, Gibbs free + feasibility table