Brain plasticity 3.2
1/47
Earn XP
Description and Tags
Brain plasticity 3.2
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
What is brain plasticity
called neuroplasticity, refers to the brain's ability to change, adapt, and reorganize itself throughout life in response to experiences, learning, or injury.
Neurogenesis
production of new neurons
starts at week 16 after conception
Stem cells
similar to progenitor cells
divides and produce neurons
found in the hippocampus
Neural Migration
(Neurons Traveling to Their Destination)
starts at week 16 after conception
After being generated, new neurons must move to the correct area in the brain or spinal cord.
Synaptogenesis
(Forming Synapses)
Starts at birth (week 40 after conception)
is the process by which synapses (connections between neurons) are formed.
This allows neurons to communicate with each other.
Axon Growth
From birth
Axons, the long, wire-like projections of neurons, grow and extend to connect with other neurons or target tissues.
This process allows the nervous system to establish communication pathways.
Oligodendrogenesis
From birth
(Oligodendrocyte Formation):
Oligodendrogenesis is the process by which oligodendrocytes, a type of glial cell, are formed in the central nervous system (CNS) - myelinating neurons - speed up the signal transmissions
Myelination of White Matter Fibers:
starts 3 months after birth
Myelination is the process where myelin, a fatty substance, wraps around axons to form a protective layer called the myelin sheath.
Synaptic pruning
(Eliminating Unused Synapses):
starts 5 months after birth
is the process where excess or unused synapses (connections between neurons) are eliminated.
This helps refine neural circuits and improve brain efficiency.
What is Neurogenesis (from PP)
Occurs when stem cells, found in the dentate gyrus, hippocampus and prefrontal cortex, divide into two cells: a stem cell and a cell that will become a neuron.
These new neurons then migrate to different areas, where they are needed
What are critical periods?
It is more sensitive, more prepared
Which areas are all crucial for neurogenesis (the creation of new neurons) in the adult brain
hippocampus (dentate gyrus)
olfactory bulb
subventricular zone (SVZ) of the lateral ventricles
WHO definition of neuroplasticity
the ability of cells of the nervous system to regenerate anatomically and functionally after being subjected to pathological environmental or developmental influences, including trauma and disease.
Neural plasticity is evident during circuit development.
NEUROPLASTICITY POSSESSES STRUCTURAL, BIOCHEMICAL AND FUNCTIONAL MECHANISMS TO CHANGE OR ADAPT TO THE SITUATION OR TO COMPENSATE CHANGES OR INJURIES.
Neuroplasticity in adults brain
Learn new abilities
Memory
Recover functions lost due to injury
Synapses and plasticity
Synapses constantly changing in response to neural activity and other influences, and show many forms of plasticity that vary in timescale from milliseconds to years.
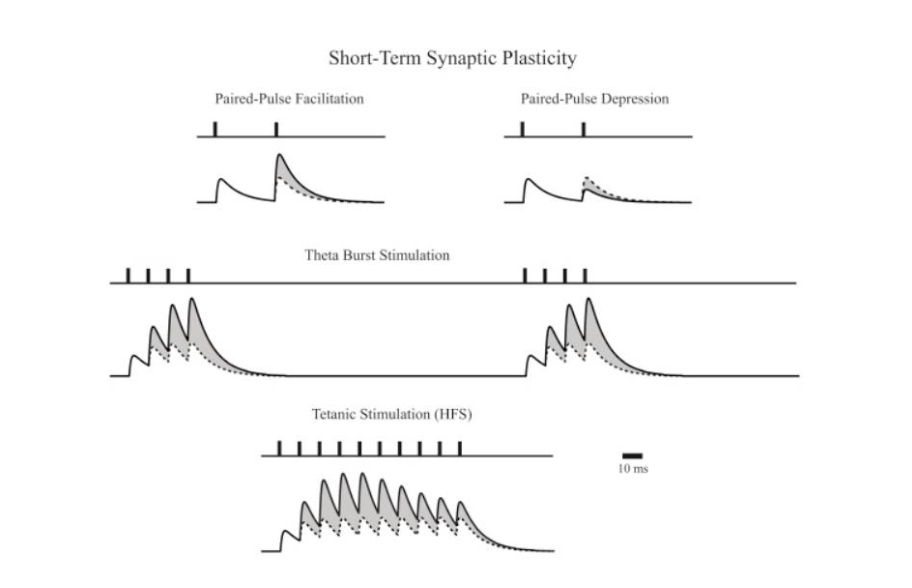
Short-term plasticity
Long-term plasticity
Short-term plasticity
seconds to minutes
Facilitation
Depression
Posttetanic Potentiation
Provide rapid but transient changes on the basis of neuronal signalling (Ca2+) and synaptic vesicle pools.
Long-term plasticity
hours to days or more
Protein phosphorylation
Changes in gene expression
Persistent changes in synaptic strength
The short-term forms of plasticity—those lasting for a few minutes or less
are readily observed during repeated activation of any chemical synapse
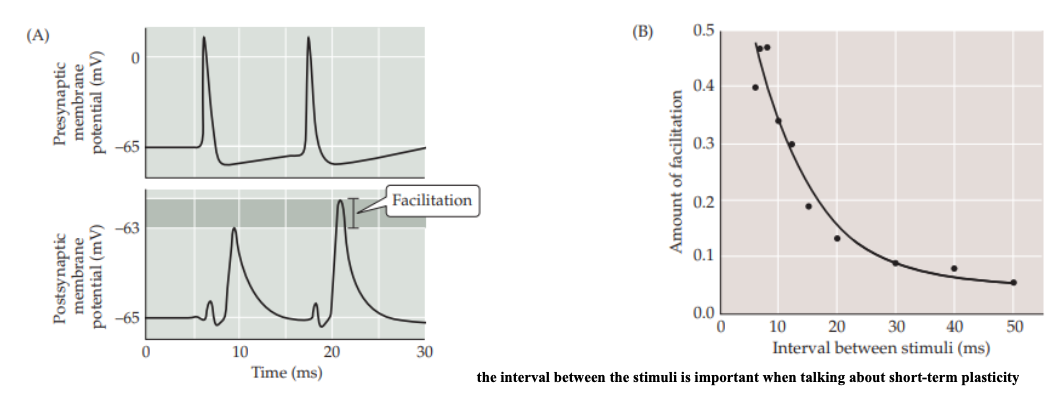
Synaptic Facilitation
is a rapid increase in synaptic strength that occurs when two or more action potentials invade the presynaptic terminal within a few milliseconds of each other.
Increased Residual Calcium in STP Facilitation
With successive action potentials, there's an accumulation of calcium in the presynaptic terminal.
This residual calcium enhances the probability of neurotransmitter vesicle release for subsequent action potentials.
Vesicle Priming and Release in STP Facilitation
Facilitation can involve the priming of additional vesicles for release, where vesicles that were not initially in the readily releasable pool become more readily available due to calcium-dependent processes.
Enhanced Exocytosis Machinery in STP Facilitation
The molecular machinery involved in vesicle fusion and exocytosis can become more efficient or activated, leading to a higher release probability.
Modulation of Presynaptic Release Sites in STP Facilitation
There might be changes in the number or sensitivity of release sites at the presynaptic terminal, allowing for more neurotransmitter to be released per action potential
Depression STP
Causes neurotransmitter release to decline during sustained synaptic activity.
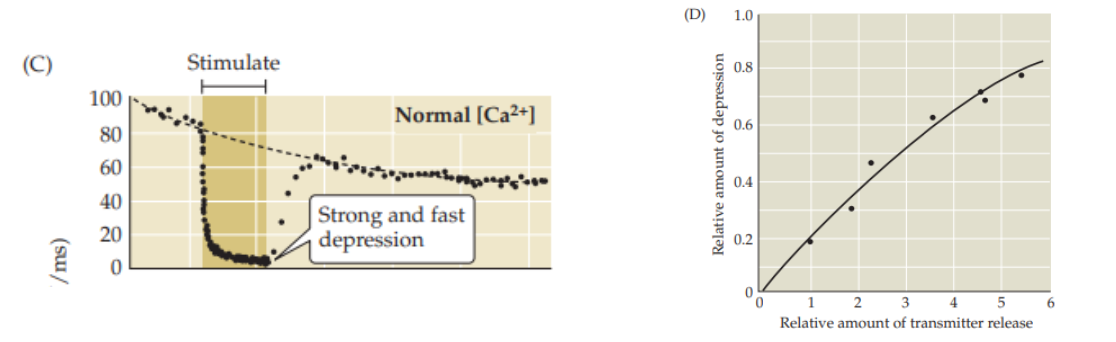
Depletion of Neurotransmitter Vesicles STP Depression
With repetitive firing of the presynaptic neuron, the readily releasable pool of neurotransmitter vesicles can become depleted.
As these vesicles are used up more quickly than they can be replenished, the amount of neurotransmitter released per action potential decreases.
Receptor Desensitization in STP Depression
Continuous or high-frequency stimulation can lead to desensitization of postsynaptic receptors (like AMPA or NMDA receptors), reducing their responsiveness to neurotransmitters, thus weakening the synaptic signal.
Changes in Presynaptic Calcium Dynamics in STP Depression
The influx of calcium into the presynaptic terminal, which normally triggers vesicle release, can become less efficient or reduced after repeated stimulation, lowering the probability of vesicle exocytosis.
Synaptic Fatigue in STP Depression
The synapse might enter a state of fatigue where the machinery for vesicle docking, fusion, or recycling does not operate at peak efficiency, contributing to depression.
Potentiation and augmentation in STP
Are elicited by repeated synaptic activity and serve to increase the amount of transmitter released from presynaptic terminals.
Both augmentation and potentiation enhance the ability of incoming Ca2+ to trigger fusion of synaptic vesicles.
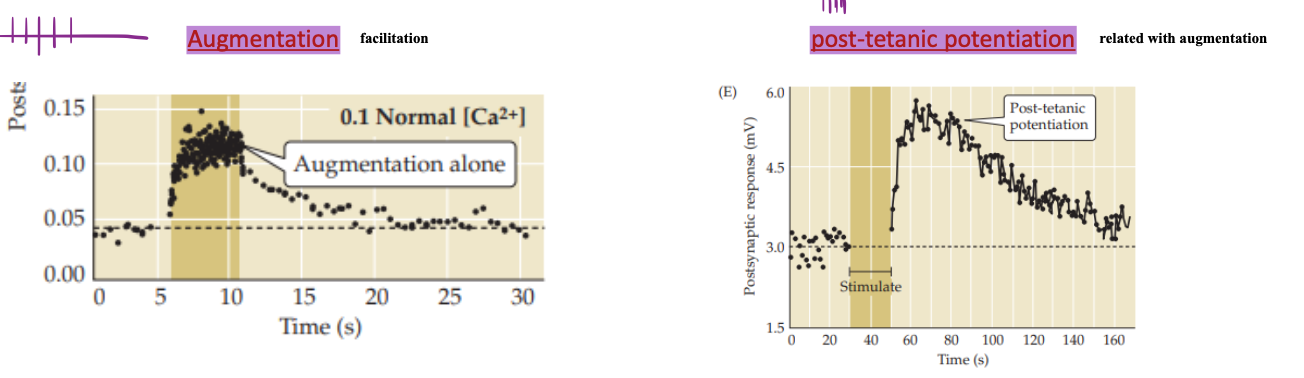
Time Scale in STP Potentiation and augmentation
Facilitation has a much shorter duration compared to Augmentation.
Facilitation is almost immediate and transient, while augmentation extends the enhancement over minutes.
Calcium Dynamics in STP Potentiation and augmentation
Both involve calcium, but Facilitation is more about the immediate, fast effects of calcium on vesicle release, whereas Augmentation might involve longer-lasting changes in calcium pools or slower calcium-dependent processes affecting vesicle handling.
Mechanistic Depth in STP Potentiation and augmentation
Facilitation is more straightforward, focusing on the presynaptic terminal's immediate response to calcium.
Augmentation suggests a more complex interplay, possibly involving different pools or pathways for calcium utilization or even postsynaptic changes.
Purpose in STP Potentiation and augmentation
Facilitation is geared towards enhancing the response to rapid, successive stimuli, aiding in fast neural computations.
Augmentation might play a role in maintaining an elevated synaptic efficacy (working better)
STP photo
Protein phosphorylation = Protein Phosphorylation in LTP
A key mechanism through which signals are transduced within neurons, affecting their function and structure.
In the context of long-term plasticity, phosphorylation of specific proteins can:
Modify Synaptic Strength
Regulate Protein Activity
Signal Cascade Activation
Modify Synaptic Strength = Protein Phosphorylation in LTP
Phosphorylation can alter the function of receptors (like NMDA and AMPA receptors), ion channels, and signaling proteins, directly affecting synaptic transmission and plasticity.
For example, phosphorylation of the AMPA receptor subunit GluR1 can increase receptor insertion into the synaptic membrane, enhancing synaptic strength.
Regulate Protein Activity = Protein Phosphorylation in LTP
Enzymes, transcription factors, and cytoskeletal proteins involved in synaptic remodeling can be activated or inactivated by phosphorylation, leading to changes in synaptic structure and function over time.
Signal Cascade Activation = Protein Phosphorylation in LTP
Phosphorylation events often trigger cascades of intracellular signaling pathways that are crucial for initiating and maintaining long-term potentiation (LTP) or long-term depression (LTD), the cellular mechanisms of long-term plasticity.
Changes in Gene Expression in LTP
Long-term plasticity requires not just immediate changes in synaptic function but also structural changes that persist over time, often necessitating new protein synthesis.
→ Changes in gene expression are fundamental here:
Transcriptional Activation
Protein Synthesis
Structural Changes
Transcriptional Activation = Changes in Gene Expression in LTP
During LTP or LTD, transcription factors like CREB (cAMP response element-binding protein) can be phosphorylated.
→ leading to their activation and subsequent binding to DNA, promoting the transcription of genes involved in synaptic growth, neurotransmitter release, and receptor function.
Protein Synthesis = Changes in Gene Expression in LTP
The transcription of genes leads to the synthesis of new proteins that can modify synaptic connections.
For instance, the synthesis of new receptors, synaptic proteins, or neurotrophic factors can stabilize changes in synaptic strength
Structural Changes = Changes in Gene Expression in LTP
Gene expression changes can lead to the production of proteins that alter the cytoskeleton, synaptic morphology, or even lead to the formation of new synapses, which are essential for long-term memory storage
Plasticity studies in invertebrates = Sea slug: Aplysia
It was suggested that modification of synaptic strength is responsible for much of the ongoing change in synaptic function in adults.
Aplysia
Habituation process
Sensitisation process
Synaptic Depression (Decrease the ability of stimuli to facilitate a response)
Habituation process in LTP
Glutamatergic activity between sensory and motor neurons is decreased.
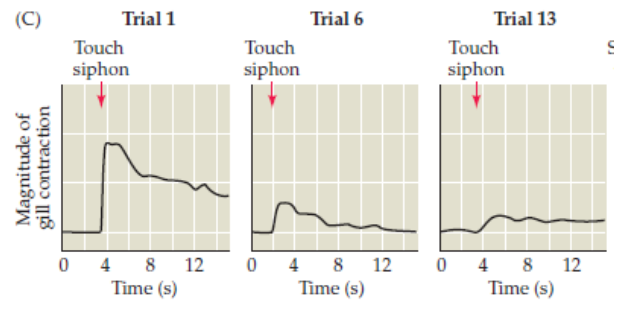
Synaptic Depression (Decrease the ability of stimuli to facilitate a response) in LTP
Reduction in the number of synaptic vesicles available for release, which reduces the amount of glutamate released.
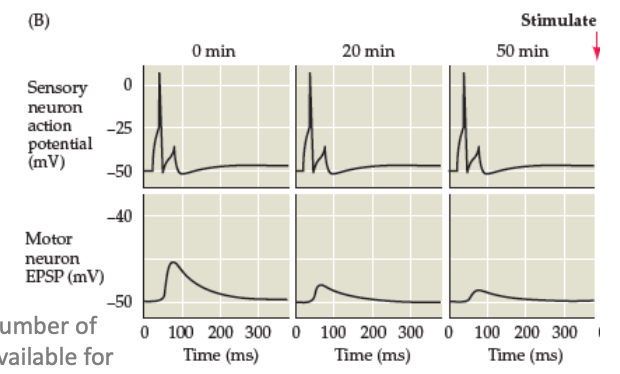
Sensitisation process in LTP
Increased recruitment of additional neurons and increased glutamatergic neurotransmission
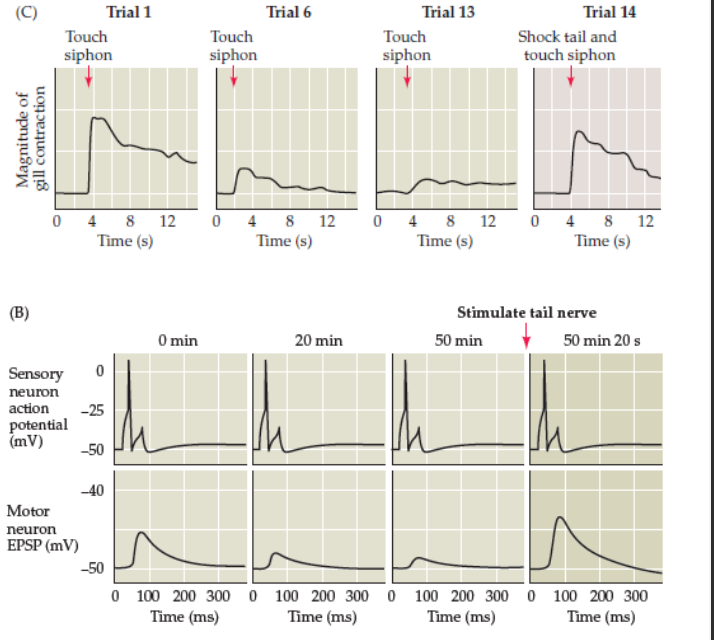
Sea slug: Aplysia results in LTP
the brain of the Sea slug: Aplysia adapted it self to the shock signals which were used.
Gradually the Sea slug: Aplysia had less and less shock
After some days the Sea slug: Aplysia was used to the shock, and did react differently “on reaction”