covalent bonds quiz
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
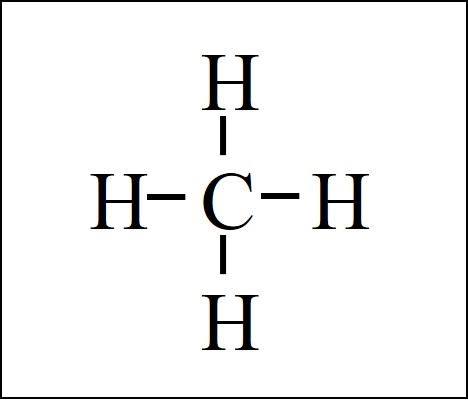
methane (\text{CH}_4)
8e-
carbon (4a) + hydrogen (1a) * 4
tetrahedral geometry
bond angle 109.5 degrees
hybrid sp3
non-polar
sigma: 4
pi: 0
2
New cards
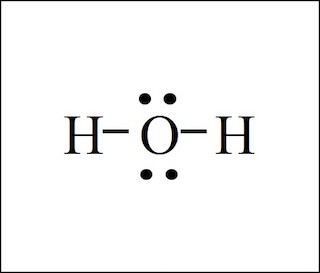
water (\text{H}_2\text{O})
8e-
hydrogen (1a) * 2 + oxygen (6a)
bent geometry
bond angle 104.5 degrees
hybrid sp3
polar
sigma: 2
pi: 0
3
New cards
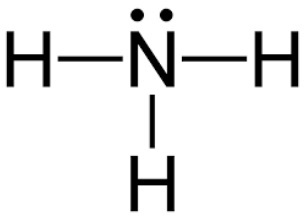
ammonia (\text{NH}_3)
8e-
nitrogen (5a) + hydrogen (1a) * 3
trigonal pyramidal geometry
bond angle 107 degrees
hybrid sp3
polar
sigma: 3
pi: 0
4
New cards
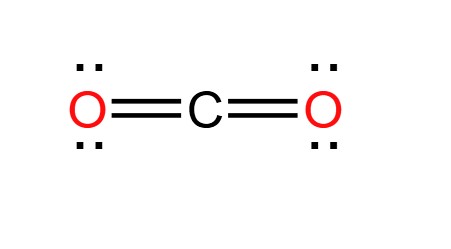
carbon dioxide (\text{CO}_2)
16e-
carbon (4a) + oxygen (6a) * 2
linear geometry
bond angle 180 degrees
hybrid sp
non-polar
sigma: 2
pi: 2