BLD 204 Exam 2
1/119
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
120 Terms
immunity
protection against infection. Freedom from a harmful condition. The system is made up of the cells and molecules that defend the body against pathogens
-recognition of nonself
-highly specific response to nonself
-memory
characteristics of human immunity
antigens
molecules recognized as nonself, the part recognized is called an epitope. They can be endogenous (native to the host) or exogenous (environmental)
innate immunity
immunity made up of cells and molecules that are always ready to provide a defense against invaders. Not antigen specific
adaptive immunity
immunity made up of cells and molecules that can respond to the "microbes that go away" from innate protection. Specific and powerful. Humoral (antibody) and cell mediated
Primary lymphoid tissue
includes the thymus and bone marrow. Cells differentiate and mature into lymphocytes here
secondary lymphoid tissue
includes the lymph nodes, spleen and malt. Adaptive immune responses develop here
-epithelial barriers
-pH
-phagocytes
-NK cells
-circulating proteins (complement, etc...)
what are innate components?
natural killer cells
cytotoxic lymphocytes which recognize loss of MHC class I "self" molecules on cells that are malignant or virus infected. They produce cytokines
T cells
cells that are thymus derived, early in life the thymus shrinks after puberty. They are found in the blood, spleen and around arterioles and lymph node inter-follicular zones. They don't detect free antigens, they have to be presented to them by MHC molecules. They are involved in cell mediated immunity
cytotoxic T cells (CD8+)
T cells involved in cell mediated immunity. MCH class I presents antigen to these cells. The CD8+ T is activated and interacts with cell that presents the antigen. It stimulates apoptosis in the infected cell an also produces and releases cytokines
Helper T cells (CD4+)
T cells that are for antibody response (humoral mediated response) working along with antigen presenting cells and B cells. They release cytokines which help stimulate B cells to make antibodies and encourage macrophages to destroy microbes.
T cell receptors
receptors that are membrane bound. They are two peptide chains that together form a binding site that recognizes a unique antigen. The variability is created by rearrangements of gene segments called V,D,J
-presented on all nucleated cells
-signal to CD8+ cytotoxic T cells
-heavy alpha chain and beta2 microglobulin chain
what type of cells have MHC class I? What type of cell do they signal to? What type of chains are they made of?
-presented on macrophages, dendritic cells and B cells
-signal to CD4+ helper T cells
-two peptide chains of more equal size
what type of cells have MHC class II? what type of cell do they signal to? What type of chains are they made of?
gamma delta T cells
T cells that have a different T cell receptor, it is not alpha beta. There is no CD4 or CD8. Don't seem to recognize protein epitopes, they recognize lipids instead. Their numbers are relatively low, mostly located in the mucosa.
B cell
cells that are bone marrow derived. They act in humoral immunity producing antibodies (as plasma cells). Found in bone marrow, circulation, spleen, MALT and lymph node follicles. They recognize antigen in many forms, don't require a MHC presentation. They recognize the antigen via BCR and take it in and process it then present it via MHC II molecules to helper T cells
B cell receptors
receptors that have unique antigen specificity. IgM is part of it. The diversity of the Ig present on different B cells is the result of gene rearrangement in V(D)J genes as in T cell receptors.
antibodies (immunoglobulins)
these molecules are one monomeric unit of 2 identical heavy chains and two identical light chains coming together to form 2 AB binding sites. There are five classes based on heavy chain structure and how many monomeric units they have. There are only two types of light chains. They agglutinate and lyse bacteria, opsonize bacteria, initiate classical complement pathway, neutralize toxins, block microbe entry into tissues and assist in killing infected cells. Not all types do all of these things
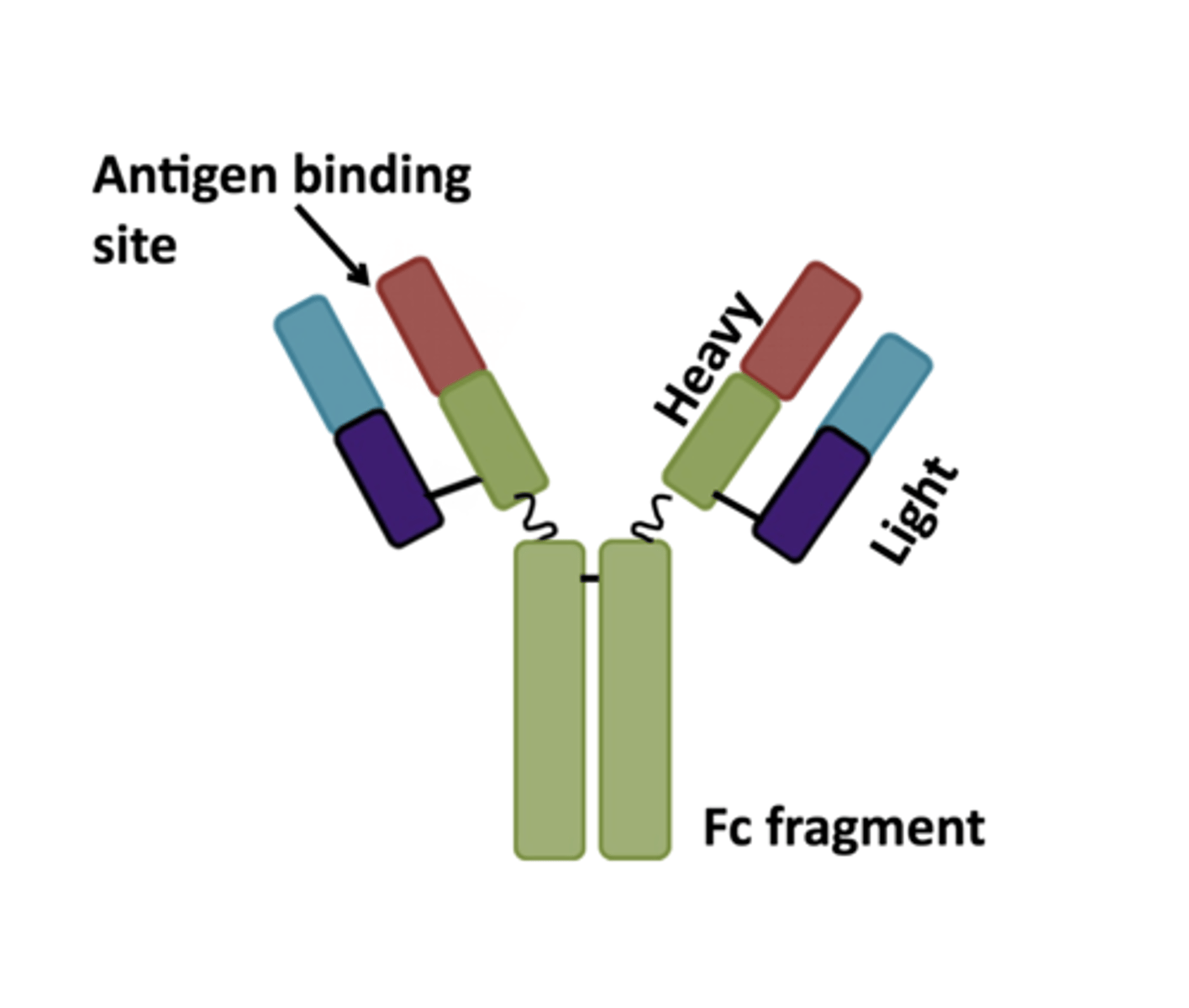
kappa or lamba
what are the two types of light chains possible in a antibody?
IgG
class of antibodies that is 70-80% of plasma immunoglobulin. It is monomeric. It diffuses out of the vasculature. It neutralizes toxins and opsonizes bacteria. It is the only class the crosses the placenta, so it can provide immunity to the baby from the mother
IgM
class of antibodies that is the largest structurally. It is a pentamer. It is units joined together at Fc fragments by J chain. It has ten binding sites so it is good for repeating epitopes. It is the first antibody that appears after immunization
IgA
class of antibodies that is found in mucosa, tears, sweat, bile and breast milk. It is a dimer with J chain connection. It stops bacteria and viruses from entering the epithelium
IgE
class of antibodies that has an Fc fragment that binds to receptors on mast cells. The antigen binding results in release of granules (histamine, leukotrienes) and calls eosinophils. It is also important in parasitic infections. It is involved in hypersensitivity
IgD
class of antibodies that present on some B cells. They help with antigen recognition/activation along with IgM. Involved in bronchial protection
Antigen presenting cells
cells that capture and display the antigen "flag." There are three types: DC dendritic cells, macrophages and B cells
dendritic cells
cells that capture and present antigens to T cells. They express MHC class II and T cell costimulatory molecules. They are found in the T cell zones in lymphoid tissue
innate: TNF, IL-1, chemokines
adaptive: IL-2, IL-4, IL-5, IL-6, IL-10, IFN-gamma
cytokines involved in immunity
somatic recombination
generates diversity of BCR and TCR antigen binding sites during lymphocyte development. VDJ gene rearrangement products are transcribed and translated into protein to become the heavy chains. Rearrangement of the light chain genes (VJ) are transcribed and translated into light chains
IL-2
what does proliferation of T cells require? (cytokines)
T independent B cell activation
activation of B cells that stimulates proliferation of B cell line, differentiation into plasma cells and antibody production. The antibodies are IgM. It doesn't result in memory. Multiple copies of the antigen are presented together and engage multiple BCRs on a cell surface
T dependent B cell activation
activation of B cells that occurs because most antigens aren't able to activate B cells. They need helper T cells to develop a response. The BCR recognizes an antigen, the antigen is ingested and chopped up and pieces are displayed on the MHC II on the surface of the B cell, then helper T cells recognize that presented antigen. T helper cell then produces cytokines that stimulate B cell proliferation and differentiation.
IL-4 and IL-2
cytokines from helper T cells that stimulate proliferation (clonal expansion) of B cell
IL-4, IL-6, IL-10, and IFN-gamma
cytokines from helper T cells that stimulate differentiation of B cells into antibody producing cells.
plasma cells
differentiated B cells that produce antibodies that have the same specificity as that original BCR. VDJ doesn't change but there is a fine tuning process for antigen affinity
memory cells
long lived cells that are ready to respond. Anamnestic response (has already encountered that antigen)
Class (Isotype) Switching
The change in the antibody class that a B cell produces. The helper T cells tell this to happen, it happens in response to cytokines. The constant region of the heavy chain (of IgM) is changed to another type. The antigen specificity remains the same. Switch to IgG (Th1), IgE (Th2) or IgA is dependent on cytokines. This occurs because we don't want IgM because its low affinity
innate, secondary, primary
what is the order of rates of immune responses from fastest to slowest?
-hypersensitivity
-autoimmunity
-immune deficiency
-neoplastic changes in immune cells
what are the four major types of immune disorders?
hypersensativity
this occurs when the secondary immune reaction is excessive and hurts the organism. There are four types of this, type I, II, III and IV
Type I hypersensitivity (allergy, immediate, reagin-activated)
type of hypersensitivity. Th2, IgE and mast cells are central to this response. During the first exposure to an antigen/allergen the Th2 helper cells are activated and influence class switching to IgE production. Lots of IgE is produced if the person is susceptible and IgE binds to mast cells via the Fc portion (sensitized). During the second exposure to the antigen there are clinical symptoms. Mast cells release mediators from granules and synthesize other from lipids (histamine, proteases, prostoglandins, leukotrienes), they also secrete cytokines in the late phase (TNF, chemokines, IL-4, IL-5, IL-13). Typical symptoms are hay fever, asthma, conjunctivitis, dermatitis, hives, GI upset, anaphylaxis
allergic response
what type of response is type I hypersensitivity?
type II hypersensitivity
cytotoxic hypersensitivity or antibody mediated. The antibody against some tissue component epitope is key here. The antibody binds to the epitope and there are three outcomes that lead to destruction: phagocytosis, complement activation and antibody dependent dysfunction. The antibody can cause a dysfunction of a cell type by blocking or stimulating receptor activity. Clinical outcomes depend on the antibody specificity, some examples are immune hemolysis, drug induced (drug acts as a carrier for a hapten, drug unmasked something that antibody could bind) and Grave's Disease
cytotoxic/antibody mediated
what type of response is type II hypersensitivity?
immune complex mediated
what type of response is type III hypersensitivity?
type III hypersensitivity
type of hypersensitivity that is immune-complex mediated. Antigen/antibody complexes are the main problem. The size of the complexes influence the outcomes. The amount of antigen and antibody matters too. Most of the time when these complexes form its no big deal unless there is lots of them and they get deposited into the tissue. Large complexes usually get cleared pretty well, lots of antibody and the deposit is usually local and lots of antigen and the depositing is more widespread because the smaller complexes are more soluble. Once deposition happens that causes inflammation (~10 days after antigen). It also causes complement activation, vasoactive enzymes and platelet aggregation. There is vascular smooth muscle damage (fibrinoid necrosis). Examples: glomerulonephritis if deposition is there, arthritis, serum sickness.
-glomerulonephritis if deposition is there
-arthritis if deposition is in the joints
-serum sickness: large amounts of foreign proteins for passive immunization, specific antibody formed, complexes with circulating antigen and its deposition in various tissues
examples of type III hypersensitivity
-immune hemolysis: hemolytic disease of the newborn due to Rh
-drug induced:drug acts as a carrier for a hapten, drug unmasked something that antibody could bind
-Grave's Disease
examples of type II hypersensitivity
delayed type hypersensitivity (cell mediated, sensitized T cells do the damage)
what type of response is type IV hypersensitivity?
Type IV hypersensitivity
type of hypersensitivity that is cell mediated, sensitized T cells do the damage. It is delayed type hypersensitivity. An example is the reaction in a TB test. happens 12-48 hours after exposure. T effector cells secrete cytokines. Prolonged response becomes granulomatous inflammation. Cytotoxic lymphocytes kill cells showing the antigen. This happens in rejection of transplants.The outcomes can be inflammation (contact dermatitis), granulomatous inflammation and tissue/organ rejection.
tolerance
lack of response to an antigen
self tolerance
the normal lack of response from the immune system to self antigens. It is developed through central tolerance and peripheral tolerance.
central tolerance
tolerance that arises when self reactive T and B cells are detected early on. The T cells undergo apoptosis during maturation in the thymus if they react to self-proteins presented to them. Some self reactive B cells also die during maturation and others undergo a second rearrangement of VDJ for a chance to become normal, if not they die too.
peripheral tolerance
type of tolerance built by self reactive Ts that escape the thymus being stopped by anergy, regulatory T cells and apoptosis related to fas death receptors
anergy
lack of costimulatory molecules on tissues that may present self results in inactivation of Ts that interact with these self tissues.
seclusion
some tissues exclude the immune cells all together so self reaction is not a problem unless the tissue is damaged and the proteins are released. Ex: lens of the eye
-previously secluded tissue proteins are exposed
-alteration of tissue antigens (infection, injury, environmental insults, drugs, etc)
-exposure of shared determinants
-impaired T cell regulation
mechanisms of autoimmune damage
shared determinants
exogenous antigens similar to self antigens
systemic lupus erythematosus
autoantibodies: classic are ANA, antinuclear antibodies. Immune complex deposition causes organ damage (especially in the skin, joints, kidney). Most common in females, genetic predisposition (HLA, DR2, DR3). Most common presentation is in a youngish female, butterfly rash on face, fever, pain and swelling in joints, chest pain and photosensitivity. As immune complexes build up, damage can manifest as nephritis, skin lesions, arthritis. There can also be hematologic and neurologic problems. Makes one more prone to infection/ May have flares and remissions. Death is usually caused by renal failure, heart problems or infections
hashimotos :(
autoantibodies to thyroid tissues lead to destruction of thyroid epithelial cells. It is T cell mediated, CD8+ cell death and ADCC. Symptoms are enlarged thyroid, hypothyroidism, usually in middle aged women, may have other autoimmune diseases as well
rheumatoid arthritis
autoimmune joint disease. Immune complex deposition and damage from cytokines. 3-5x more likely in women, 20-40 years old. Genetic and environmental factors play a role.
-inadequate response to an antigen
-hypersensitivity
-autoimmune
-neoplasms of immune cells
what are the major problems of immune disorders?
Afferent Immune Deficiency
immune deficiency that has problematic antigen presentation and recognition
Efferent Immune Deficiency
immune deficiency that has problematic T cell activation and antibody production
afferent and efferent
what are the types of specific immune deficiency?
problems with complement and phagocytosis
what are types of nonspecific immune deficiency?
primary immunodeficiency
immune deficiency that is congenital, genetically determined, rare and appears earlier in life
secondary immunodeficiency
immune deficiency that is acquired and is usually secondary to a therapy or other disease
-severe combined immunodeficiency (SCID)
-DiGeorge syndrome
-Bruton disease
examples of primary immune deficiencies
Severe Combined Immunodeficiency (SCID)
primary immune deficiency that affects both the T and B cell functions. The thymus is hypoplastic, other lymphoid tissue is atrophic. No antibodies and no cell mediated responses leads to an infant with severe, recurrent infection often due to opportunistic pathogens. Common genetic defects that lead to it are an ADA deficiency which leads to a buildup of A and dATP which leads to lymphocyte death and a decrease in DNA synthesis. Also a common gamma chain defect can lead to defective receptors for cytokines which leads to proliferation and decrease of T and B cell precursors. The treatment for this is bone marrow transplant.
DiGeorge Syndrome
primary immune defect where there is a thymic development defect (lack T cells). The common genetic defect for it is deletion on chromosome 22. Often viruses and intracellular bacterial infections occur, sometime fungus and protozoa too. it can sometimes get better by age 5.
Bruton disease
primary immune deficiency, also known as X linked agammaglobulinemia. Pre B cells fail to mature and get into the blood. there will be low plasma immunoglobulin. often repeated infections by bacteria P. carinii and Giardia. T cell mediated immunity should be normal
secondary immune deficiency
immune deficiency that is acquired. Possible causes are age, malnutrition, neoplasia of immune system, immune suppression and infection. Ex: AIDS
AIDS
acquired immune deficiency syndrome. Caused by a viral infection which is HIV. Depletion of CD4+ lymphocytes which leads to serious immunosuppression which leads to opportunistic infections, neoplasms and neurological problems. It is spread through contact with blood or body fluids that contain the virus. Two major targets are the immune system and the central nervous system. It causes a loss of CD4+ T cell loss, defective DC and macrophage function, damage to lymphoid tissue (later). Some neoplasms that result from it are Kaposi's sarcoma, lymphoma of the brain and cervical cancer
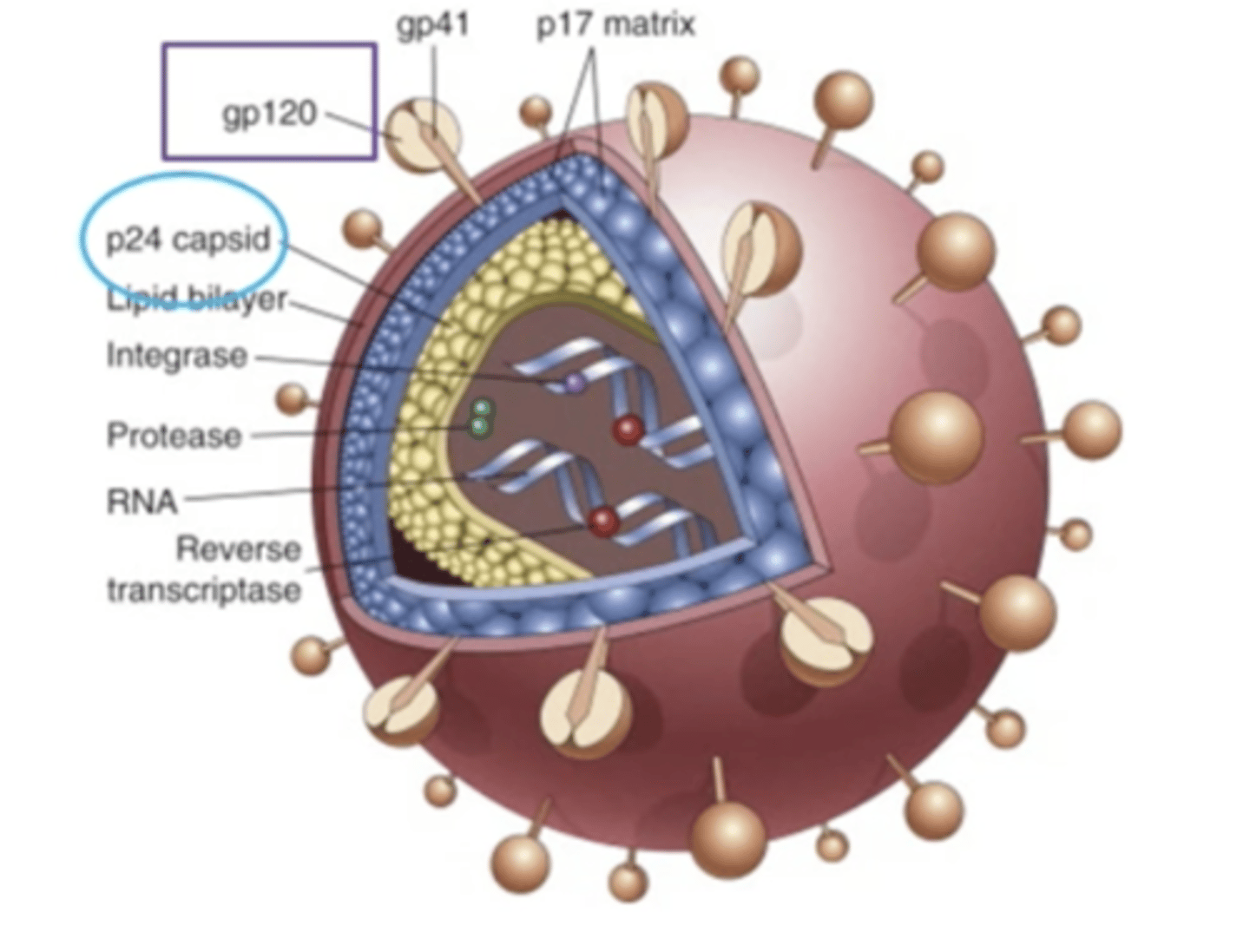
cells that express CD4, Th cells, macrophages, dendritic cells, binding CD4 is not sufficient for infection
what is the tropism for AIDS? (cell types virus needs to invade)
CCR5
chemokine receptor on macrophages, monocytes and T cells, a mutation in this receptor could be protective against HIV/AIDS infection
CXCR4
chemokine receptor on T cells that once it lets in the HIV/AIDS virus, the virus becomes especially virulent. It contributes to T cell depletion at the end of the virus
overtime the R5 infecting virus's gp120 mutates to infect via the R4
what is one type of AIDS mutation?
Primary HIV infection
-lymphoid tissue infection established
-viremia (flu like symptoms), partial control by CTLs and antibodies developed, latency/low levels of infection
-infection/cytokines encourage viral replication, CD4+ cell/lymphoid tissue destruction
gp120
protein on the HIV viral envelope that attaches to the CD4 cell and chemokine receptors
loss of precursor cells/infection of them. chronic activation by HIV antigen apoptosis. direct cell lysis by virus. killing by CTLs
how do CD4+ T cells die during an AIDS infection
-macrophage/monocyte lineage microglial cells infected
-probably gets there through infected monocytes
-neurons are not infected
-actual damage not well understood
CNS damage from AIDS
amyloidosis
extracellular proteins deposited as fibrils made of beta plated sheets. Macroscopically it makes an organ look larger, firmer and paler. Microscopically there is lack of structure, eosinophilia, binding congo red dye, and bundle of fibers with electron microscopy
primary amyloidosis
Deposition of amyloid without any concurrent or previous disease. Monoclonal plasma cell proliferation. Multiple Myeloma
secondary amyloidosis
amyloidosis that follows identifiable disease, often chronic inflammation. Ex: Rheumatoid arthritis
Immune Origin Amyloidosis
AL amyloid light chain type. Protein precursor: Ig light chain (intact light chain, amino terminal of light chain, usually lambda). source: single B lineage clone. symptoms: neuropathy, restrictive cardiomyopathy, skin manifestations, polyarthopathy, macroglossia, factor X deficiency, carpal tunnel syndrom
Hemodialysis-Associated Amyloidosis
Aβ2M Type. Protein precursor: β2 Microglobulin. Source: Shed from cell membranes due to dialysis. Outcomes: carpal tunnel syndrome
Reactive Systemic Amyloidosis
secondary amyloidosis. Protein precursor: serum amyloid A (SAA) which is produced by the liver usually in small amounts, following inflammation it rises (1000 fold), signals for up-regulation (IL-1, TNF alpha, IL-6). Symptoms: systemic distribution of deposits, kidneys, liver, spleen, adrenal, main cause of death is renal failure
hereditary systemic amyloidosis
protein precursor: transthyretin with mutation. source: plasma. its normally already at beta plated sheet. Normal and mutated formed together form fibrils. two types: neuropathic and cardiopathic.
Localized amyloidosis
Endocrine-related.
-Precursor: short portion of calcitonin molecule. Thyroid carcinoma and pancreatic islet cells in Type II diabetes.
Intracerebral
-Alzheimer's : A4. Prion associated diseases: prion protein
transplantation
replacing an organ with another or portion of another. The best results are if you don't stimulate an immune response. HLA matching is a major contributing factor to protection from rejection.
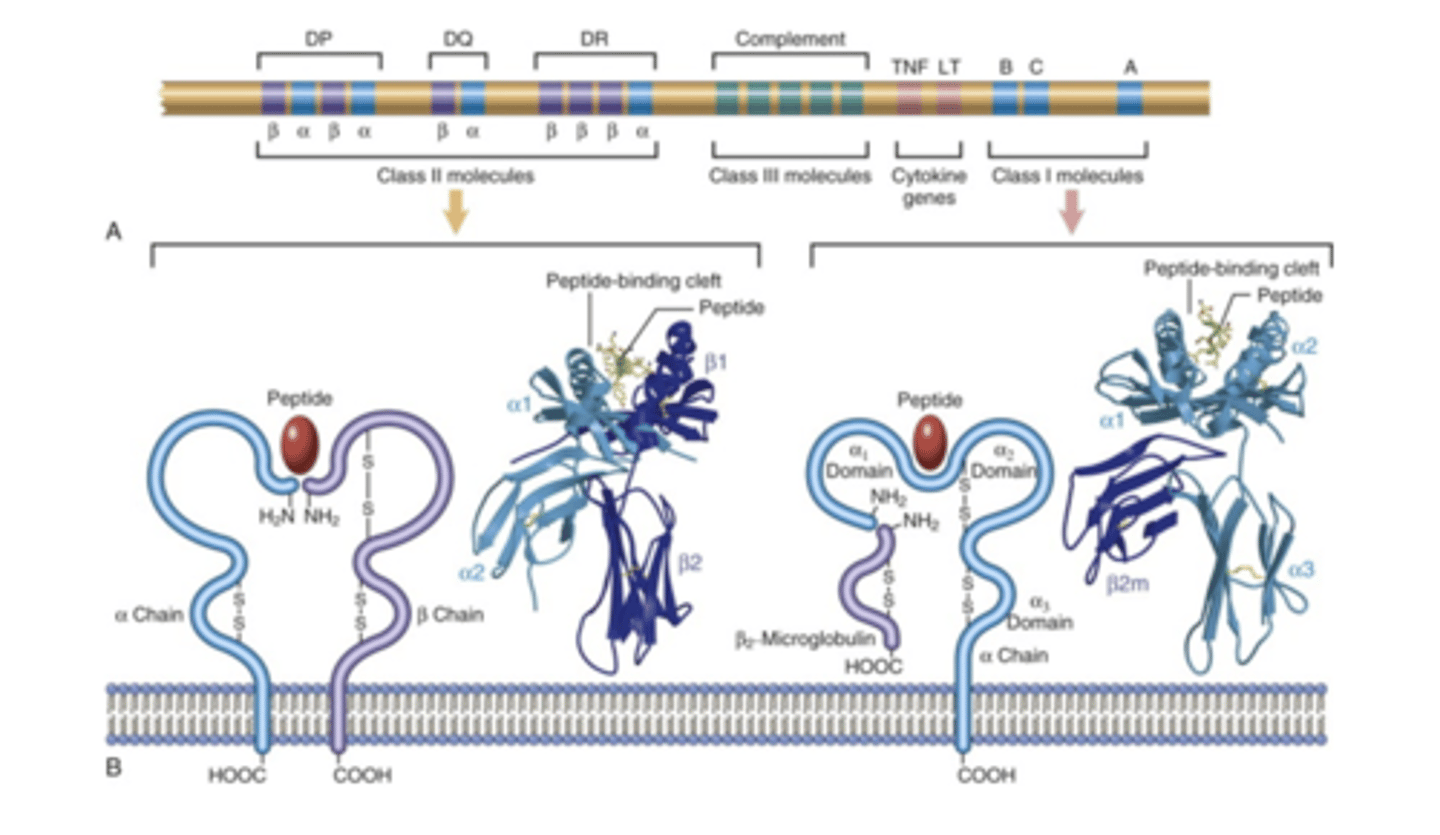
human leukocyte antigen
the human versions of the Major histocompatibility complex genes. You inherit one allele of each gene from each parent. Multiple copies of each gene in each population, it is highly polymorphic. Each person has a unique combination of these molecules expressed on their cells.
autograft
transplantation of healthy tissue from one site to another site in the same individual
isograft
transplant between identical twins
allograft
transplantation of healthy tissue from one person to another person
xenograft
transplantation (dermis only) from a foreign donor (usually a pig) and transferred to a human
direct recognition
this type of recognition occurs between a host T cell and a donor APC. There is activation of the host CD8+ T cells to CTLs (MHC I). CD4+ may be activated as well (MHCII).
indirect recognition
recognition occurs when host APC presents donor antigen to host T cells. The CD4+ cells activate B cells that make antibodies against graft (cytokine damage too).
-Class I and II genes
-histocompatibility lab looks for typing HLA genes through serological, molecular or cellular testing
what does HLA matching involve?
serological testing
histocompatibility lab test that looks for antibody/antigen interactions between donor and recipient. Tested through HLA molecules on the surface of cells.